Recent achievements in nano-based technologies for ocular disease diagnosis and treatment, review and update
- PMID: 35918688
- PMCID: PMC9344723
- DOI: 10.1186/s12951-022-01567-7
Recent achievements in nano-based technologies for ocular disease diagnosis and treatment, review and update
Abstract
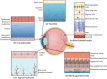
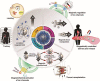
Ocular drug delivery is one of the most challenging endeavors among the various available drug delivery systems. Despite having suitable drugs for the treatment of ophthalmic disease, we have not yet succeeded in achieving a proper drug delivery approach with the least adverse effects. Nanotechnology offers great opportunities to overwhelm the restrictions of common ocular delivery systems, including low therapeutic effects and adverse effects because of invasive surgery or systemic exposure. The present review is dedicated to highlighting and updating the recent achievements of nano-based technologies for ocular disease diagnosis and treatment. While further effort remains, the progress illustrated here might pave the way to new and very useful ocular nanomedicines.
Keywords: Diagnosis; Nanotechnology; Ocular diseases; Treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Nanomedicines for back of the eye drug delivery, gene delivery, and imaging.Prog Retin Eye Res. 2013 Sep;36:172-98. doi: 10.1016/j.preteyeres.2013.04.001. Epub 2013 Apr 17. Prog Retin Eye Res. 2013. PMID: 23603534 Free PMC article. Review.
-
Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 Jul;11(4):e1548. doi: 10.1002/wnan.1548. Epub 2018 Dec 3. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 30506871 Review.
-
Nano-based eye drop: Topical and noninvasive therapy for ocular diseases.Adv Drug Deliv Rev. 2023 Mar;194:114721. doi: 10.1016/j.addr.2023.114721. Epub 2023 Feb 10. Adv Drug Deliv Rev. 2023. PMID: 36773886 Review.
-
Nanotechnology in ocular drug delivery.Drug Discov Today. 2008 Feb;13(3-4):144-51. doi: 10.1016/j.drudis.2007.10.021. Drug Discov Today. 2008. PMID: 18275912 Review.
-
Drug delivery methods based on nanotechnology for the treatment of eye diseases.Ann Med Surg (Lond). 2023 Oct 17;85(12):6029-6040. doi: 10.1097/MS9.0000000000001399. eCollection 2023 Dec. Ann Med Surg (Lond). 2023. PMID: 38098602 Free PMC article. Review.
Cited by
-
Harnessing Nanoparticles to Overcome Antimicrobial Resistance: Promises and Challenges.Curr Pharm Des. 2025;31(4):292-306. doi: 10.2174/0113816128326718240809091654. Curr Pharm Des. 2025. PMID: 39219123 Review.
-
Nanomedicine in glaucoma treatment; Current challenges and future perspectives.Mater Today Bio. 2024 Sep 4;28:101229. doi: 10.1016/j.mtbio.2024.101229. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39296355 Free PMC article. Review.
-
Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders.Pharmaceuticals (Basel). 2023 Jan 10;16(1):96. doi: 10.3390/ph16010096. Pharmaceuticals (Basel). 2023. PMID: 36678597 Free PMC article. Review.
-
Recent advancements in nanomaterial-laden contact lenses for diagnosis and treatment of glaucoma, review and update.J Nanobiotechnology. 2023 Nov 2;21(1):402. doi: 10.1186/s12951-023-02166-w. J Nanobiotechnology. 2023. PMID: 37919748 Free PMC article. Review.
-
In vivo and in vitro efficacy of the ithmid kohl/zinc-oxide nanoparticles, ithmid kohl/Aloe vera, and zinc-oxide nanoparticles/Aloe vera for the treatment of bacterial endophthalmitis.Sci Rep. 2024 Jul 8;14(1):15746. doi: 10.1038/s41598-024-66341-1. Sci Rep. 2024. PMID: 38977762 Free PMC article.
References
-
- Nagaraj R, Bijukumar DR, Mathew B, Scott EA, Mathew MT. A review on recent advancements in ophthalmology devices: currently in market and under clinical trials. J Drug Deliv Sci Technol. 2019;52:334–345.
-
- Balantrapu T. Latest global blindness & VI prevalence figures published in Lancet. 2018. www.iapb.org/news/latest-global-blindness-vi-prevalence-figures-publishe.... Accessed 5 Jan 2017.
-
- Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology. 2001;108(3):563–571. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

