Safety and tolerance assessment of milk fat globule membrane-enriched infant formulas in healthy term Chinese infants: a randomised multicenter controlled trial
- PMID: 35918695
- PMCID: PMC9347101
- DOI: 10.1186/s12887-022-03507-8
Safety and tolerance assessment of milk fat globule membrane-enriched infant formulas in healthy term Chinese infants: a randomised multicenter controlled trial
Abstract
Background: Milk fat globule membrane (MFGM), natural to breast milk, is essential for neonatal development, but lacking from standard infant formulas.
Objectives: To evaluate the safety and tolerability of MFGM supplementation in formula for infants 0 to 12 months.
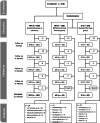
Methods: In a prospective, multicentre, double-blind, randomized trial, healthy term infants were randomized to a standard formula (SF, n = 104) or an MFGM-enriched formula (MF, n = 108) for 6 months and a corresponding follow-on formula until 12 months. Exclusively breast-fed infants (n = 206) were recruited as the reference group (BFR). Tolerance and safety events were recorded continuously. Anthropometric measurements were assessed at enrolment, 42 days and 4, 6, 8 and 12 months.
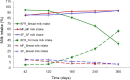
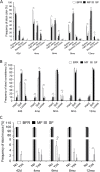
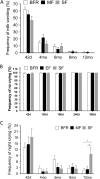
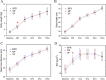
Results: Infants (n = 375) completed the study with average dropout of < 20%. Stool frequency, color, and consistency between SF and MF were not significantly different throughout, except the incidence of loose stools in MF at 6 months being lower than for SF (odds ratio 0.216, P < 0.05) and the frequency of green-colored stools at 12 months being higher in MF (CI 95%, odds ratio 8.92, P < 0.05). The BFR had a higher frequency of golden stools and lower rate of green stools (4-6 months) than the two formula-fed groups (P < 0.05). SF displayed more diarrhoea (4.8%) than MF (1%) and BFR (1%) at the 8-month visit (P < 0.05). BFR (0-1%) had significantly less (P < 0.05) lower respiratory infections than MF (4.6-6.5%) and SF (2.9-5.8%) at 6- and 8-months, respectively. Formula intake, frequency of spit-up/vomiting or poor sleep were similar between SF and MF. Growth rate (g/day) was similar at 4, 6, 8 and 12 months between the 3 groups, but growth rate for BFR was significantly higher than for SF and MF at 42 days (95% CI, P = 0.001).
Conclusions: MFGM-enriched formula was safe and well-tolerated in healthy term infants between 0 and 12 months, and total incidences of adverse events were similar to that for the SF group. A few differences in formula tolerance were observed, however these differences were not in any way related to poor growth.
Keywords: Formula; Formula tolerance; Growth; Infant feeding; Milk fat globule membrane; Neurodevelopment.
© 2022. The Author(s).
Conflict of interest statement
Angela Rowan is an employee of Fonterra Co-operative Group Limited. Sophie Gallier was previously employed by Fonterra Co-operative Group Limited. The other authors declare no competing interests.
Figures
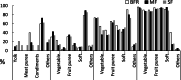
Similar articles
-
Infant formula supplemented with milk fat globule membrane compared with standard infant formula for the cognitive development of healthy term-born formula-fed infants: protocol for a randomised controlled trial.BMJ Open. 2024 Jul 1;14(6):e083399. doi: 10.1136/bmjopen-2023-083399. BMJ Open. 2024. PMID: 38951000 Free PMC article.
-
Neurodevelopmental outcomes of healthy Chinese term infants fed infant formula enriched in bovine milk fat globule membrane for 12 months - A randomized controlled trial.Asia Pac J Clin Nutr. 2021 Sep;30(3):401-414. doi: 10.6133/apjcn.202109_30(3).0007. Asia Pac J Clin Nutr. 2021. PMID: 34587700 Clinical Trial.
-
Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial.Am J Clin Nutr. 2014 Apr;99(4):860-8. doi: 10.3945/ajcn.113.064295. Epub 2014 Feb 5. Am J Clin Nutr. 2014. PMID: 24500150 Clinical Trial.
-
Milk Fat Globule Membrane Supplementation in Children: Systematic Review with Meta-Analysis.Nutrients. 2021 Feb 24;13(3):714. doi: 10.3390/nu13030714. Nutrients. 2021. PMID: 33668227 Free PMC article.
-
Milk protein-based infant formula containing rice starch and low lactose reduces common regurgitation in healthy term infants: a randomized, blinded, and prospective trial.J Am Coll Nutr. 2014;33(2):136-46. doi: 10.1080/07315724.2013.828578. J Am Coll Nutr. 2014. PMID: 24724771 Review.
Cited by
-
Tolerance of Infants Fed a Hydrolyzed Rice Infant Formula with 2'-Fucosyllactose (2'-FL) Human Milk Oligosaccharide (HMO).Nutrients. 2024 Jun 13;16(12):1863. doi: 10.3390/nu16121863. Nutrients. 2024. PMID: 38931218 Free PMC article.
-
Infant formula supplemented with milk fat globule membrane compared with standard infant formula for the cognitive development of healthy term-born formula-fed infants: protocol for a randomised controlled trial.BMJ Open. 2024 Jul 1;14(6):e083399. doi: 10.1136/bmjopen-2023-083399. BMJ Open. 2024. PMID: 38951000 Free PMC article.
-
Functional Significance of Probiotic Bacterial Interactions with Milk Fat Globules in a Human Host.Microorganisms. 2025 Jan 21;13(2):223. doi: 10.3390/microorganisms13020223. Microorganisms. 2025. PMID: 40005590 Free PMC article. Review.
-
Supplemented Infant Formula and Human Breast Milk Show Similar Patterns in Modulating Infant Microbiota Composition and Function In Vitro.Int J Mol Sci. 2024 Feb 2;25(3):1806. doi: 10.3390/ijms25031806. Int J Mol Sci. 2024. PMID: 38339084 Free PMC article.
-
Milk Fat Globule Membranes for Mental Health across the Human Lifespan.Foods. 2024 May 24;13(11):1631. doi: 10.3390/foods13111631. Foods. 2024. PMID: 38890860 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

