Candidate genes for infertility: an in-silico study based on cytogenetic analysis
- PMID: 35918717
- PMCID: PMC9347124
- DOI: 10.1186/s12920-022-01320-x
Candidate genes for infertility: an in-silico study based on cytogenetic analysis
Abstract
Background: The cause of infertility remains unclear in a significant proportion of reproductive-age couples who fail to conceive naturally. Chromosomal aberrations have been identified as one of the main genetic causes of male and female infertility. Structural chromosomal aberrations may disrupt the functioning of various genes, some of which may be important for fertility. The present study aims to identify candidate genes and putative functional interaction networks involved in male and female infertility using cytogenetic data from cultured peripheral blood lymphocytes of infertile patients.
Methods: Karyotypic analyses was done in 201 infertile patients (100 males and 101 females) and 201 age and gender matched healthy controls (100 males and 101 females) after 72 h peripheral lymphocyte culturing and GTG banding, followed by bioinformatic analysis using Cytoscape v3.8.2 and Metascape.
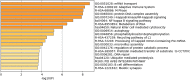
Results: Several chromosomal regions with a significantly higher frequency of structural aberrations were identified in the infertile males (5q2, 10q2, and 17q2) and females (6q2, 16q2, and Xq2). Segregation of the patients based on type of infertility (primary v/s secondary infertility) led to the identification of chromosomal regions with a significantly higher frequency of structural aberrations exclusively within the infertile males (5q2, 17q2) and females (16q2) with primary infertility. Cytoscape identified two networks specific to these regions: a male specific network with 99 genes and a female specific network with 109 genes. The top enriched GO terms within the male and female infertility networks were "skeletal system morphogenesis" and "mRNA transport" respectively. PSME3, PSMD3, and CDC27 were the top 3 hub genes identified within the male infertility network. Similarly, UPF3B, IRF8, and PSMB1 were the top 3 hub genes identified with the female infertility network. Among the hub genes identified in the male- and female-specific networks, PSMB1, PSMD3, and PSME3 are functional components of the proteasome complex. These hub genes have a limited number of reports related to their respective roles in maintenance of fertility in mice model and humans and require validation in further studies.
Conclusion: The candidate genes predicted in the present study can serve as targets for future research on infertility.
Keywords: Female infertility; In-silico; Karyotyping; Male infertility; Network analysis; Protein–protein interaction.
© 2022. The Author(s).
Conflict of interest statement
Corresponding author, Vasudha Sambyal and co-author, Kamlesh Guleria are Associate Editors of the journal BMC Medical Genomics. Rest of the authors declare that they have no competing interests.
Figures



Similar articles
-
Prevalence of Chromosomal Abnormalities in Iranian Patients with Infertility.Arch Iran Med. 2023 Feb 1;26(2):110-116. doi: 10.34172/aim.2023.17. Arch Iran Med. 2023. PMID: 37543931 Free PMC article.
-
Cytogenetic studies in patients with reproductive failure.Acta Obstet Gynecol Scand. 2003 Jan;82(1):53-6. doi: 10.1034/j.1600-0412.2003.820109.x. Acta Obstet Gynecol Scand. 2003. PMID: 12580840
-
Cytogenetic Abnormalities Found in Patients with Reproductive Problems.Med Arch. 2017 Dec;71(6):396-399. doi: 10.5455/medarh.2017.71.396-399. Med Arch. 2017. PMID: 29416198 Free PMC article.
-
[Value of karyotyping women patients of couples referred for sterility].Gynecol Obstet Fertil. 2003 Jan;31(1):66-9. doi: 10.1016/s1297-9589(02)00008-5. Gynecol Obstet Fertil. 2003. PMID: 12659787 Review. French.
-
Genetics of human male infertility.Singapore Med J. 2009 Apr;50(4):336-47. Singapore Med J. 2009. PMID: 19421675 Review.
Cited by
-
Transcriptional Differences in Identical Twins With Different Reproductive Capacities: A Case Report.Cureus. 2023 Jun 23;15(6):e40847. doi: 10.7759/cureus.40847. eCollection 2023 Jun. Cureus. 2023. PMID: 37492809 Free PMC article.
-
Survey of Structural Autosomal Abnormalities and Autosomal Variants in Infertile Patients Treated at Some IVF Centers in Vietnam.Appl Clin Genet. 2025 Apr 11;18:29-40. doi: 10.2147/TACG.S510933. eCollection 2025. Appl Clin Genet. 2025. PMID: 40235466 Free PMC article.
-
Discordant effects of maternal age on the human MII oocyte transcriptome.Mol Hum Reprod. 2025 Jul 3;31(3):gaaf038. doi: 10.1093/molehr/gaaf038. Mol Hum Reprod. 2025. PMID: 40729310 Free PMC article.
-
Fertility problems in men carrying chromosome 7 inversion: A retrospective, observational study.Medicine (Baltimore). 2025 Jan 17;104(3):e41358. doi: 10.1097/MD.0000000000041358. Medicine (Baltimore). 2025. PMID: 39833054 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

