Tick-borne encephalitis affects sleep-wake behavior and locomotion in infant rats
- PMID: 35918749
- PMCID: PMC9344439
- DOI: 10.1186/s13578-022-00859-7
Tick-borne encephalitis affects sleep-wake behavior and locomotion in infant rats
Abstract
Background/aims: Tick-borne encephalitis (TBE) is a disease affecting the central nervous system. Over the last decade, the incidence of TBE has steadily increased in Europe and Asia despite the availably of effective vaccines. Up to 50% of patients after TBE suffer from post-encephalitic syndrome that may develop into long-lasting morbidity. Altered sleep-wake functions have been reported by patients after TBE. The mechanisms causing these disorders in TBE are largely unknown to date. As a first step toward a better understanding of the pathology of TBEV-inducing sleep dysfunctions, we assessed parameters of sleep structure in an established infant rat model of TBE.
Methods: 13-day old Wistar rats were infected with 1 × 106 FFU Langat virus (LGTV). On day 4, 9, and 21 post infection, Rotarod (balance and motor coordination) and open field tests (general locomotor activity) were performed and brains from representative animals were collected in each subgroup. On day 28 the animals were implanted with a telemetric EEG/EMG system. Sleep recording was continuously performed for 24 consecutive hours starting at day 38 post infection and visually scored for Wake, NREM, and REM in 4 s epochs.
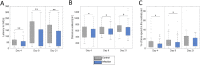
Results: As a novelty of this study, infected animals showed a significant larger percentage of time spend awake during the dark phase and less NREM and REM compared to the control animals (p < 0.01 for all comparisons). Furthermore, it was seen, that during the dark phase the wake bout length in infected animals was prolonged (p = 0.043) and the fragmentation index decreased (p = 0.0085) in comparison to the control animals. LGTV-infected animals additionally showed a reduced rotarod performance ability at day 4 (p = 0.0011) and day 9 (p = 0.0055) and day 21 (p = 0.0037). A lower locomotor activity was also seen at day 4 (p = 0.0196) and day 9 (p = 0.0473).
Conclusion: Our data show that experimental TBE in infant rats affects sleep-wake behavior, leads to decreased spontaneous locomotor activity, and impaired moto-coordinative function.
Keywords: Anxiety-like behavior; Chemokines and cytokines; Infant rats; Langat virus; Locomotion; Neurofilament; Sleep; Sleep–wake behavior; Tick-borne encephalitis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
A tick-borne encephalitis model in infant rats infected with langat virus.J Neuropathol Exp Neurol. 2014 Dec;73(12):1107-15. doi: 10.1097/NEN.0000000000000131. J Neuropathol Exp Neurol. 2014. PMID: 25383637
-
Tick-borne encephalitis - a review of current epidemiology, clinical symptoms, management and prevention.Przegl Epidemiol. 2020;74(2):316-325. doi: 10.32394/pe.74.24. Przegl Epidemiol. 2020. PMID: 33115220
-
Sleep-Wake and Circadian Disorders after Tick-Borne Encephalitis.Microorganisms. 2022 Jan 27;10(2):304. doi: 10.3390/microorganisms10020304. Microorganisms. 2022. PMID: 35208759 Free PMC article. Review.
-
Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines.Antiviral Res. 2019 Apr;164:23-51. doi: 10.1016/j.antiviral.2019.01.014. Epub 2019 Jan 31. Antiviral Res. 2019. PMID: 30710567 Review.
-
Type I interferon protects mice from fatal neurotropic infection with Langat virus by systemic and local antiviral responses.J Virol. 2014 Nov;88(21):12202-12. doi: 10.1128/JVI.01215-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122777 Free PMC article.
References
-
- Dörrbecker B, Dobler G, Spiegel M, Hufert FT. Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med Infect Dis. 2010;8(4):213–222. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

