Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry
- PMID: 35919181
- PMCID: PMC9301632
- DOI: 10.1039/d2ra02848d
Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry
Abstract
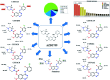
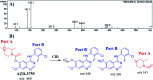
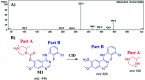
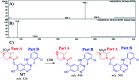
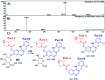
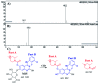
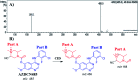
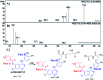
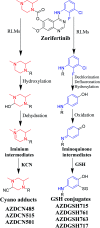
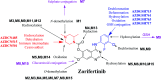
Zorifertinib (AZD-3759; ZFB) is a potent, novel, oral, small molecule used for the treatment of non-small cell lung cancer (NSCLC). ZFB is Epidermal Growth Factor Receptor (EGFR) inhibitor that is characterized by good permeability of the blood-brain barrier for (NSCLC) patients with EGFR mutations. The present research reports the profiling of in vitro, in vivo and reactive metabolites of ZFB. Prediction of vulnerable metabolic sites and reactivity pathways (cyanide and GSH) of ZFB were performed by WhichP450™ module (StarDrop software package) and XenoSite reactivity model (XenoSite Web Predictor-Home), respectively. ZFB in vitro metabolites were done by incubation with isolated perfused rat liver hepatocytes and rat liver microsomes (RLMs). Extraction of ZFB and its related metabolites from the incubation matrix was done by protein precipitation. In vivo metabolism was performed by giving ZFB (10 mg kg-1) through oral gavage to Sprague Dawley rats that were housed in metabolic cages. Urine was collected at specific time intervals (0, 6, 12, 18, 24, 48, 72, 96 and 120 h) from ZFB dosing. The collected urine samples were filtered then stored at -70 °C. N-Methyl piperazine ring of ZFB undergoes phase I metabolism forming iminium intermediates that were stabilized using potassium cyanide as a trapping agent. Incubation of ZFB with RLMs were performed in the presence of 1.0 mM KCN and 1.0 mM glutathione to check reactive intermediates as it is may be responsible for toxicities associated with ZFB usage. For in vitro metabolites there were six in vitro phase I metabolites, three in vitro phase II metabolites, seven reactive intermediates (four GSH conjugates and three cyano adducts) of ZFB were detected by LC-IT-MS. For in vivo metabolites there were six in vivo phase I and three in vivo phase II metabolites of ZFB were detected by LC-IT-MS. In vitro and in vivo phase I metabolic pathways were N-demethylation, O-demethylation, hydroxylation, reduction, defluorination and dechlorination. In vivo phase II metabolic reaction was direct sulphate and glucuronic acid conjugation with ZFB.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest for the current work.
Figures
Similar articles
-
Identification and characterization of in silico, in vivo, in vitro, and reactive metabolites of infigratinib using LC-ITMS: bioactivation pathway elucidation and in silico toxicity studies of its metabolites.RSC Adv. 2020 Apr 23;10(28):16231-16244. doi: 10.1039/c9ra10871h. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498820 Free PMC article.
-
Identification and characterization of in vitro, in vivo, and reactive metabolites of tandutinib using liquid chromatography ion trap mass spectrometry.Anal Methods. 2021 Jan 28;13(3):399-410. doi: 10.1039/d0ay02106g. Anal Methods. 2021. PMID: 33410830
-
Identification and characterization of in vivo, in vitro and reactive metabolites of vandetanib using LC-ESI-MS/MS.Chem Cent J. 2018 Sep 24;12(1):99. doi: 10.1186/s13065-018-0467-5. Chem Cent J. 2018. PMID: 30251155 Free PMC article.
-
Estimation of zorifertinib metabolic stability in human liver microsomes using LC-MS/MS.J Pharm Biomed Anal. 2022 Mar 20;211:114626. doi: 10.1016/j.jpba.2022.114626. Epub 2022 Jan 29. J Pharm Biomed Anal. 2022. PMID: 35123331
-
Investigation of Fenebrutinib Metabolism and Bioactivation Using MS3 Methodology in Ion Trap LC/MS.Molecules. 2023 May 22;28(10):4225. doi: 10.3390/molecules28104225. Molecules. 2023. PMID: 37241965 Free PMC article.
Cited by
-
Analyzing the metabolic fate of oral administration drugs: A review and state-of-the-art roadmap.Front Pharmacol. 2022 Oct 7;13:962718. doi: 10.3389/fphar.2022.962718. eCollection 2022. Front Pharmacol. 2022. PMID: 36278150 Free PMC article. Review.
-
Tackling assay interference associated with small molecules.Nat Rev Chem. 2024 May;8(5):319-339. doi: 10.1038/s41570-024-00593-3. Epub 2024 Apr 15. Nat Rev Chem. 2024. PMID: 38622244 Review.
-
Recent Advances in Pretreatment Methods and Detection Techniques for Veterinary Drug Residues in Animal-Derived Foods.Metabolites. 2025 Mar 28;15(4):233. doi: 10.3390/metabo15040233. Metabolites. 2025. PMID: 40278362 Free PMC article. Review.
References
-
- Papadopoulou E. Tsoulos N. Tsirigoti A. Apessos A. Agiannitopoulos K. Metaxa-Mariatou V. Zarogoulidis K. Zarogoulidis P. Kasarakis D. Kakolyris S. Dahabreh J. Vlastos F. Zoublios C. Rapti A. Papageorgiou N. G. Veldekis D. Gaga M. Aravantinos G. Karavasilis V. Karagiannidis N. Nasioulas G. Oncol. Lett. 2015;10:2176–2184. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

