Dynamic changes of inducible nitric oxide synthase expression in rat's retina and its role on blood-retinal barrier injury after acute high intraocular pressure
- PMID: 35919326
- PMCID: PMC9318089
- DOI: 10.18240/ijo.2022.07.03
Dynamic changes of inducible nitric oxide synthase expression in rat's retina and its role on blood-retinal barrier injury after acute high intraocular pressure
Abstract
Aim: To clarify the role of inducible nitric oxide synthase (iNOS) in blood-retinal barrier (BRB) injury after acute high intraocular pressure (IOP) in rats.
Methods: Forty-two Sprague-Dawley (SD) rats were randomized into 7 groups [control (Cont), 3, 6, 12, 24, 48, and 72h, n=6]. Except Cont group, other groups' retina tissue was obtained at corresponding time points after a model of acute high IOP have been established in rats. The expression of iNOS and tight junction protein zonula occludens (ZO)-1 was detected by Western blotting. Evans blue (EB; 3% ) was injected into the great saphenous vein to detect the leakage of EB by spectrophotometer. Nine rats were divided into Cont, 6h, 12h groups, the expression of iNOS was localized by immunofluorescence. In order to verify the role of iNOS in the damage to BRB, thirty-six rats were randomly divided into 4 groups [Cont, Cont+inhibitor (Inh), 6h and 6h+Inh, n=9]. After treatment with the iNOS-specific inhibitor 1400W, the expression of iNOS and ZO-1 and the leakage of BRB were detected again.
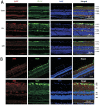
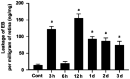
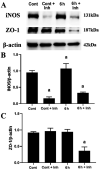
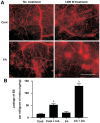
Results: The immunofluorescence results showed that the expression of iNOS was observed in the Cont group and 6h group, but not in the 12h group. iNOS was mainly expressed in the retinal nerve fiber layer, ganglion cell layer and inner nuclear layer and that it did not colocalize with the retinal ganglion cell marker NeuN but was co-expressed with the vascular endothelial cell marker CD31. Western blotting showed that in the early period (3h, 6h) after acute high IOP, the expression of iNOS was upregulated, then the down-regulation of iNOS were tested in the follow-up timing spots. ZO-1 expression showed a continuous down-regulation after 6h. The quantitative results for EB showed that the amount of EB leakage began to increase at 3h after acute high IOP. At 6h, the leakage of EB was lower, but at 12h, the leakage of EB was highest, after which it gradually recovered but remained higher than that in the Cont group. The expression of iNOS was down-regulated after 1400W treatment. ZO-1 expression was not significantly changed in the Cont+Inh group and the 6h group, and significantly down-regulated in the 6h+Inh group, and the leakage of EB was significantly increased after 1400W treatment.
Conclusion: These results suggest that the upregulation of iNOS expression in the early stage after acute high IOP may have a protective effect on BRB injury.
Keywords: ZO-1; acute high intraocular pressure; blood-retinal barrier; inducible nitric oxide synthase.
International Journal of Ophthalmology Press.
Figures
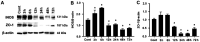
Similar articles
-
Vascular endothelial growth factor-165b protects the blood-retinal barrier from damage after acute high intraocular pressure in rats.Int J Ophthalmol. 2022 Aug 18;15(8):1231-1239. doi: 10.18240/ijo.2022.08.02. eCollection 2022. Int J Ophthalmol. 2022. PMID: 36017048 Free PMC article.
-
Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy.Invest Ophthalmol Vis Sci. 2007 Nov;48(11):5257-65. doi: 10.1167/iovs.07-0112. Invest Ophthalmol Vis Sci. 2007. PMID: 17962481
-
Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia-reperfusion.Brain Res. 2011 Jul 27;1403:67-77. doi: 10.1016/j.brainres.2011.06.005. Epub 2011 Jun 12. Brain Res. 2011. PMID: 21704308
-
Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin.Clin Exp Ophthalmol. 2019 Dec;47(9):1182-1197. doi: 10.1111/ceo.13619. Epub 2019 Sep 15. Clin Exp Ophthalmol. 2019. PMID: 31483932
-
[The role of hydrogen sulfide in acute lung injury during endotoxic shock and its relationship with nitric oxide and carbon monoxide].Zhonghua Yi Xue Za Zhi. 2008 Aug 19;88(32):2240-5. Zhonghua Yi Xue Za Zhi. 2008. PMID: 19087669 Chinese.
Cited by
-
Impaired pericyte-Müller glia interaction via PDGFRβ suppression aggravates photoreceptor loss in a rodent model of light-induced retinal injury.Int J Ophthalmol. 2024 Oct 18;17(10):1800-1808. doi: 10.18240/ijo.2024.10.05. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430007 Free PMC article.
References
-
- Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Retinal ganglion cell death in glaucoma: exploring the role of neuroinflammation. Eur J Pharmacol. 2016;787:134–142. - PubMed
-
- Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. - PubMed
LinkOut - more resources
Full Text Sources
