Supramolecular assembly confined purely organic room temperature phosphorescence and its biological imaging
- PMID: 35919429
- PMCID: PMC9278158
- DOI: 10.1039/d2sc01770a
Supramolecular assembly confined purely organic room temperature phosphorescence and its biological imaging
Abstract
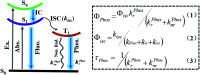
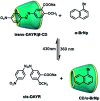
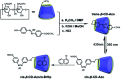
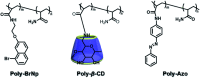
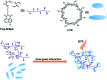
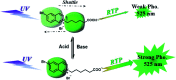
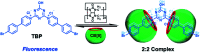
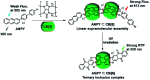
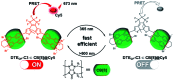
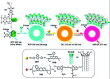
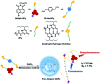
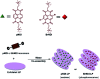
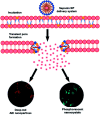
Purely organic room temperature phosphorescence, especially in aqueous solution, is attracting increasing attention owing to its large Stokes shift, long lifetime, low preparation cost, low toxicity, good processing performance advantages, and broad application value. This review mainly focuses on macrocyclic (cyclodextrin and cucurbituril) hosts, nanoassembly, and macromolecule (polyether) confinement-driven RTP. As an optical probe, the assembly and the two-stage assembly strategy can realize the confined purely organic RTP and achieve energy transfer and light-harvesting from fluorescence to delayed fluorescence or phosphorescence. This supramolecular assembly is widely applied for luminescent materials, cell imaging, and other fields because it effectively avoids oxygen quenching. In addition, the near-infrared excitation, near-infrared emission, and in situ imaging of purely organic room temperature phosphorescence in assembled confinement materials are also prospected.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







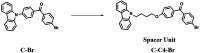








Similar articles
-
Supramolecular Purely Organic Room-Temperature Phosphorescence.Acc Chem Res. 2021 Sep 7;54(17):3403-3414. doi: 10.1021/acs.accounts.1c00336. Epub 2021 Aug 17. Acc Chem Res. 2021. PMID: 34403251
-
Noncovalent Polymerization-Activated Ultrastrong Near-Infrared Room-Temperature Phosphorescence Energy Transfer Assembly in Aqueous Solution.Adv Mater. 2022 Sep;34(38):e2203534. doi: 10.1002/adma.202203534. Epub 2022 Aug 17. Adv Mater. 2022. PMID: 35771589
-
Enhancing Purely Organic Room Temperature Phosphorescence via Supramolecular Self-Assembly.Adv Mater. 2024 May;36(18):e2311922. doi: 10.1002/adma.202311922. Epub 2024 Feb 5. Adv Mater. 2024. PMID: 38270348 Review.
-
Two-Photon Excited Near-Infrared Phosphorescence Based on Secondary Supramolecular Confinement.Adv Sci (Weinh). 2022 Jun;9(18):e2201182. doi: 10.1002/advs.202201182. Epub 2022 Apr 24. Adv Sci (Weinh). 2022. PMID: 35466559 Free PMC article.
-
Cyclodextrin supramolecular assembly confined luminescent materials.Chem Sci. 2024 Oct 17;15(44):18259-71. doi: 10.1039/d4sc05698a. Online ahead of print. Chem Sci. 2024. PMID: 39464618 Free PMC article. Review.
Cited by
-
An anthracene-containing crown ether: synthesis, host-guest properties and modulation of solid state luminescence.Chem Sci. 2024 Sep 18;15(39):16300-6. doi: 10.1039/d4sc05077k. Online ahead of print. Chem Sci. 2024. PMID: 39309098 Free PMC article.
-
Cucurbituril-based supramolecular host-guest complexes: single-crystal structures and dual-state fluorescence enhancement.Chem Sci. 2023 Dec 5;15(2):458-465. doi: 10.1039/d3sc04813f. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179534 Free PMC article.
-
Electrostatically mediated phosphorescence enhancement of micro-nano composites.Nat Commun. 2025 May 6;16(1):4189. doi: 10.1038/s41467-025-59360-7. Nat Commun. 2025. PMID: 40328754 Free PMC article.
-
Engineering perfluoroarenes for enhanced molecular barrier effect and chirality transfer in solutions.Chem Sci. 2025 Jan 10;16(8):3498-3508. doi: 10.1039/d4sc07859d. eCollection 2025 Feb 19. Chem Sci. 2025. PMID: 39845871 Free PMC article.
-
Aqueous up-conversion organic phosphorescence and tunable dual emission in a single-molecular emitter.Chem Sci. 2025 Mar 3;16(15):6290-6297. doi: 10.1039/d4sc08330j. eCollection 2025 Apr 9. Chem Sci. 2025. PMID: 40092596 Free PMC article.
References
-
- Li W. Wu S. Xu X. Zhuang J. Zhang H. Zhang X. Hu C. Lei B. Kaminski C. F. Liu Y. Chem. Mater. 2019;31:9887–9894. doi: 10.1021/acs.chemmater.9b04120. - DOI
- Luker G. D. Luker K. E. J. Nucl. Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. - DOI - PubMed
- Voss T. C. Demarco I. A. Day R. N. BioTechniques. 2005;38:413–424. doi: 10.2144/05383RV01. - DOI - PMC - PubMed
-
- Chan J. Dodani S. C. Chang C. J. Nat. Chem. 2012;4:973–984. doi: 10.1038/nchem.1500. - DOI - PMC - PubMed
- Ding D. Li K. Liu B. Tang B. Z. Acc. Chem. Res. 2013;46:2441–2453. doi: 10.1021/ar3003464. - DOI - PubMed
- Fan Y. Wang P. Lu Y. Wang R. Zhou L. Zheng X. Li X. Piper J. A. Zhang F. Nat. Nanotechnol. 2018;13:941–946. doi: 10.1038/s41565-018-0221-0. - DOI - PubMed
- Kenry Duan Y. Liu B. Adv. Mater. 2018;30:1802394. doi: 10.1002/adma.201802394. - DOI - PubMed
- Lei Z. Zhang F. Angew. Chem., Int. Ed. 2021;60:16294–16308. doi: 10.1002/anie.202007040. - DOI - PubMed
- Li J. Pu K. Chem. Soc. Rev. 2019;48:38–71. doi: 10.1039/C8CS00001H. - DOI - PubMed
- Lovell J. F. Liu T. W. B. Chen J. Zheng G. Chem. Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. - DOI - PubMed
- McHugh K. J. Jing L. Behrens A. M. Jayawardena S. Tang W. Gao M. Langer R. Jaklenec A. Adv. Mater. 2018;30:1706356. doi: 10.1002/adma.201706356. - DOI - PubMed
- Miao Q. Xie C. Zhen X. Lyu Y. Duan H. Liu X. Jokerst J. V. Pu K. Nat. Biotechnol. 2017;35:1102–1110. doi: 10.1038/nbt.3987. - DOI - PubMed
- van Dam G. M. Themelis G. Crane L. M. A. Harlaar N. J. Pleijhuis R. G. Kelder W. Sarantopoulos A. de Jong J. S. Arts H. J. G. van der Zee A. G. J. Bart J. Low P. S. Ntziachristos V. Nat. Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. - DOI - PubMed
- Wan H. Yue J. Zhu S. Uno T. Zhang X. Yang Q. Yu K. Hong G. Wang J. Li L. Ma Z. Gao H. Zhong Y. Su J. Antaris A. L. Xia Y. Luo J. Liang Y. Dai H. Nat. Commun. 2018;9:1171. doi: 10.1038/s41467-018-03505-4. - DOI - PMC - PubMed
- Zhang J. Zhen X. Upputuri P. K. Pramanik M. Chen P. Pu K. Adv. Mater. 2017;29:1604764. doi: 10.1002/adma.201604764. - DOI - PubMed
-
- Huang J. Li J. Lyu Y. Miao Q. Pu K. Nat. Mater. 2019;18:1133–1143. doi: 10.1038/s41563-019-0378-4. - DOI - PubMed
- Wang B. Wang Y. Wang Y. Zhao Y. Yang C. Zeng Z. Huan S. Song G. Zhang X. Anal. Chem. 2020;92:4154–4163. doi: 10.1021/acs.analchem.0c00329. - DOI - PubMed
- Zhang K. Y. Yu Q. Wei H. Liu S. Zhao Q. Huang W. Chem. Rev. 2018;118:1770–1839. doi: 10.1021/acs.chemrev.7b00425. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

