Electrochemical synthesis of N, N'-disubstituted indazolin-3-ones via an intramolecular anodic dehydrogenative N-N coupling reaction
- PMID: 35919432
- PMCID: PMC9278119
- DOI: 10.1039/d2sc01827f
Electrochemical synthesis of N, N'-disubstituted indazolin-3-ones via an intramolecular anodic dehydrogenative N-N coupling reaction
Abstract
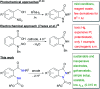
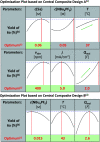
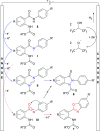
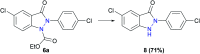
The use of electricity as a traceless oxidant enables a sustainable and novel approach to N,N'-disubstituted indazolin-3-ones by an intramolecular anodic dehydrogenative N-N coupling reaction. This method is characterized by mild reaction conditions, an easy experimental setup, excellent scalability, and a high atom economy. It was used to synthesize various indazolin-3-one derivatives in yields up to 78%, applying inexpensive and sustainable electrode materials and a low supporting electrolyte concentration. Mechanistic studies, based on cyclic voltammetry experiments, revealed a biradical pathway. Furthermore, the access to single 2-aryl substituted indazolin-3-ones by cleavage of the protecting group could be demonstrated.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Regioselective Electrochemical Construction of Csp2-Csp2 Linkage at C5-C5' Position of 2-Oxindoles via an Intermolecular Anodic Dehydrogenative Coupling.Chemistry. 2024 Dec 13;30(70):e202403420. doi: 10.1002/chem.202403420. Epub 2024 Nov 11. Chemistry. 2024. PMID: 39308393
-
Electrochemical Synthesis of Carbazoles by Dehydrogenative Coupling Reaction.Chemistry. 2020 Dec 4;26(68):15847-15851. doi: 10.1002/chem.202003430. Epub 2020 Oct 29. Chemistry. 2020. PMID: 32737905 Free PMC article.
-
Dehydrogenative Electrochemical Synthesis of N-Aryl-3,4-Dihydroquinolin-2-ones by Iodine(III)-Mediated Coupling Reaction.Chemistry. 2024 Feb 1;30(7):e202303388. doi: 10.1002/chem.202303388. Epub 2023 Dec 13. Chemistry. 2024. PMID: 38018461
-
A Decade of Electrochemical Dehydrogenative C,C-Coupling of Aryls.Acc Chem Res. 2020 Jan 21;53(1):45-61. doi: 10.1021/acs.accounts.9b00511. Epub 2019 Dec 18. Acc Chem Res. 2020. PMID: 31850730 Review.
-
Anodic Oxidation for the Stereoselective Synthesis of Heterocycles.Acc Chem Res. 2020 Jan 21;53(1):105-120. doi: 10.1021/acs.accounts.9b00513. Epub 2019 Dec 24. Acc Chem Res. 2020. PMID: 31872753 Review.
Cited by
-
Efficient and Sustainable Electrosynthesis of N-Sulfonyl Iminophosphoranes by the Dehydrogenative P-N Coupling Reaction.JACS Au. 2024 Apr 18;4(6):2188-2196. doi: 10.1021/jacsau.4c00156. eCollection 2024 Jun 24. JACS Au. 2024. PMID: 38938819 Free PMC article.
References
-
- Tse E. Butner L. Huang Y. Hall I. H. Arch. Pharm. Pharm. Med. Chem. 1996;329:35. doi: 10.1002/ardp.19963290107. - DOI - PubMed
- Abouzid K. A. M. El-Abhar H. S. Arch. Pharmacal Res. 2003;26:1. doi: 10.1002/ardp.19963290107. - DOI - PubMed
- Bruneau P. Delvare C. Edwards M. P. McMillan R. M. J. Med. Chem. 1991;34:1028. doi: 10.1002/ardp.19963290107. - DOI - PubMed
- Roth A. Ott S. Farber K. M. Palazzo T. A. Conrad W. E. Haddadin M. J. Tantillo D. J. Cross C. E. Eiserich J. P. Kurth M. J. Bioorg. Med. Chem. 2014;22:6422. doi: 10.1002/ardp.19963290107. - DOI - PMC - PubMed
- Yu W. Guo Z. Orth P. Madison V. Chen L. Dai C. Feltz R. J. Girijavallabhan V. M. Kim S. H. Kozlowski J. A. Lavey B. J. Li D. Lundell D. Niu X. Piwinski J. J. Popovici-Muller J. Rizvi R. Rosner K. E. Shankar B. B. Shih N.-Y. Siddiqui M. A. Sun J. Tong L. Umland S. Wong M. K. C. Yang D. Zhou G. Bioorg. Med. Chem. Lett. 2010;20:1877. doi: 10.1002/ardp.19963290107. - DOI - PubMed
LinkOut - more resources
Full Text Sources

