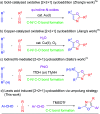
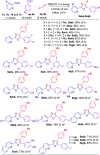
An umpolung strategy for intermolecular [2 + 2 + 1] cycloaddition of aryl aldehydes and nitriles: a facile access to 2,4,5-trisubstituted oxazoles
- PMID: 35919435
- PMCID: PMC9278343
- DOI: 10.1039/d2sc00046f
An umpolung strategy for intermolecular [2 + 2 + 1] cycloaddition of aryl aldehydes and nitriles: a facile access to 2,4,5-trisubstituted oxazoles
Abstract
We have described the first example of an umpolung strategy for intermolecular [2 + 2 + 1] cycloaddition between two aryl aldehydes and a nitrile under the influence of TMSOTf that proceeds through the formation of N-C, O-C and C-C bonds providing a simple synthetic protocol for obtaining 2,4,5-trisubstituted oxazoles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
[3 + 2] Cycloaddition/Oxidative Aromatization Sequence via Photoredox Catalysis: One-Pot Synthesis of Oxazoles from 2H-Azirines and Aldehydes.Org Lett. 2015 Aug 21;17(16):4070-3. doi: 10.1021/acs.orglett.5b01994. Epub 2015 Aug 7. Org Lett. 2015. PMID: 26250789
-
Synthesis of 2,4,5-Trisubstituted Oxazoles via Pd-Catalyzed C-H Addition to Nitriles/Cyclization Sequences.Org Lett. 2019 Apr 19;21(8):2745-2749. doi: 10.1021/acs.orglett.9b00700. Epub 2019 Apr 1. Org Lett. 2019. PMID: 30931572
-
Metal Free Access to Polysubstituted Pyrimidines via Nitrile Activation and [2+2+2] Cycloaddition.Chemistry. 2021 Dec 15;27(70):17565-17569. doi: 10.1002/chem.202103219. Epub 2021 Oct 22. Chemistry. 2021. PMID: 34626013
-
A Facile Microwave-Assisted Synthesis of Oxazoles and Diastereoselective Oxazolines Using Aryl-Aldehydes, p-Toluenesulfonylmethyl Isocyanide under Controlled Basic Conditions.ACS Omega. 2020 Oct 20;5(43):28239-28248. doi: 10.1021/acsomega.0c04130. eCollection 2020 Nov 3. ACS Omega. 2020. PMID: 33163807 Free PMC article.
-
Umpolung Strategy for α-Functionalization of Aldehydes for the Addition of Thiols and other Nucleophiles.Angew Chem Int Ed Engl. 2019 Dec 2;58(49):17856-17862. doi: 10.1002/anie.201911793. Epub 2019 Oct 24. Angew Chem Int Ed Engl. 2019. PMID: 31595649 Review.
References
-
- Breslow R. J. Am. Chem. Soc. 1958;80:3719–3726. doi: 10.1021/ja01547a064. - DOI
- Enders D. Niemeier O. Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. - DOI - PubMed
- Hopkinson M. N. Richter C. Schedler M. Glorius F. Nature. 2014;510:485–496. doi: 10.1038/nature13384. - DOI - PubMed
- Flanigan D. M. Romanov-Michailidis F. White N. A. Rovis T. Chem. Rev. 2015;115:9307–9387. doi: 10.1021/acs.chemrev.5b00060. - DOI - PMC - PubMed
- Menon R. S. Biju A. T. Nair V. Beilstein J. Org. Chem. 2016;12:444–461. doi: 10.3762/bjoc.12.47. - DOI - PMC - PubMed
-
- Grobel B.-T. Seebach D. Synthesis. 1977:357–402. doi: 10.1055/s-1977-24412. - DOI
- Seebach D. Angew. Chem., Int. Ed. 1979;18:239–258. doi: 10.1002/anie.197902393. - DOI
- Yus M. Najera C. Foubelo F. Tetrahedron. 2003;59:6147–6212. doi: 10.1016/S0040-4020(03)00955-4. - DOI
- Izquierdo J. Hutson G. E. Cohen D. T. Scheidt K. A. Angew. Chem., Int. Ed. 2012;51:11686–11698. doi: 10.1002/anie.201203704. - DOI - PMC - PubMed
- Dai X.-J. Li C.-C. Li C.-J. Chem. Soc. Rev. 2021;50:10733–10742. doi: 10.1039/D1CS00418B. - DOI - PubMed
-
-
For reviews, see:
- Blanco-Urgoiti J. Añorbe L. Pérez-Serrano L. Domínguez G. Pérez-Castells J. Chem. Soc. Rev. 2004;33:32–42. doi: 10.1039/B300976A. - DOI - PubMed
- Bonaga L. V. R. Krafft M. E. Tetrahedron. 2004;60:9795–9833. doi: 10.1016/j.tet.2004.06.072. - DOI
- Gibson S. E. Mainolfi N. Angew. Chem., Int. Ed. 2005;44:3022–3037. doi: 10.1002/anie.200462235. - DOI - PubMed
- Chatani N. Chem. Rec. 2008;8:201–212. doi: 10.1002/tcr.20149. - DOI - PubMed
- Kitagaki S. Inagaki F. Mukai C. Chem. Soc. Rev. 2014;43:2956–2978. doi: 10.1039/C3CS60382B. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

