Inclusion of older adults and reporting of consent processes in randomized controlled trials in the emergency department: A scoping review
- PMID: 35919513
- PMCID: PMC9337842
- DOI: 10.1002/emp2.12774
Inclusion of older adults and reporting of consent processes in randomized controlled trials in the emergency department: A scoping review
Abstract
Objective: Conducting research in the emergency department (ED) is often complicated by patients' acute and chronic illnesses, which can adversely affect cognition and subsequently capacity to consent for research, especially in older adults. Validated screening tools to assess capacity to consent for research exist, but neither the frequency of use nor which ones are used for ED research are known.
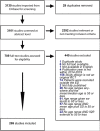
Methods: We conducted a scoping review using standard review techniques. Inclusion criteria included (1) randomized controlled trials (RCTs) from publication years 2014-2019 that (2) enrolled participants only in the ED, (3) included patients aged 65+ years, and (4) were fully published in English. Articles were sourced from Embase and screened using Covidence.
Results: From 3130 search results, 269 studies passed title/abstract and full text screening. Average of the mean or median ages was 55.7 years (SD 14.2). The mean number of study participants was 311.9 [range 8-10,807 participants]. A few (n = 13, 4.8%) waived or had exception from informed consent. Of the 256 studies requiring consent, a fourth (26.5%, n = 68) specifically excluded patients due to impaired capacity to consent. Only 11 (4.3%) documented a formal capacity screening tool and only 13 (5.1%) reported consent by legally authorized representative (LAR).
Conclusions: Most RCTs enrolling older adults in EDs did not report assessment of capacity to consent or use of LARs. This snapshot of informed consent procedures is potentially concerning and suggests that either research consent processes for older patients and/or reporting of consent processes require improvement.
Keywords: capacity; emergency department; geriatrics; older adults; research consent.
© 2022 The Authors. JACEP Open published by Wiley Periodicals LLC on behalf of American College of Emergency Physicians.
Conflict of interest statement
The authors report no conflict of interest.
Figures
Similar articles
-
The effectiveness of health literacy interventions on the informed consent process of health care users: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):82-94. doi: 10.11124/jbisrir-2015-2304. JBI Database System Rev Implement Rep. 2015. PMID: 26571285
-
Advance consent for participation in randomised controlled trials for emergency conditions: a scoping review.BMJ Open. 2023 Feb 7;13(2):e066742. doi: 10.1136/bmjopen-2022-066742. BMJ Open. 2023. PMID: 36750278 Free PMC article.
-
Older Adults, the "Social Admission," and Nonspecific Complaints in the Emergency Department: Protocol for a Scoping Review.JMIR Res Protoc. 2023 Mar 15;12:e38246. doi: 10.2196/38246. JMIR Res Protoc. 2023. PMID: 36920467 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Frailty assessment tools in the emergency department: A geriatric emergency department guidelines 2.0 scoping review.J Am Coll Emerg Physicians Open. 2023 Dec 29;5(1):e13084. doi: 10.1002/emp2.13084. eCollection 2024 Feb. J Am Coll Emerg Physicians Open. 2023. PMID: 38162531 Free PMC article.
Cited by
-
Digital Informed Consent for Older Adults in Emergency Department Research.Appl Hum Factors Ergon Conf. 2023;78:10.54941/ahfe1003449. doi: 10.54941/ahfe1003449. Appl Hum Factors Ergon Conf. 2023. PMID: 40709065 Free PMC article.
-
Improving the inclusion of an under-served group in trials: development and implementation of the INCLUDE Impaired Capacity to Consent Framework.Trials. 2024 Jan 25;25(1):83. doi: 10.1186/s13063-024-07944-x. Trials. 2024. PMID: 38273417 Free PMC article.
References
-
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. - PubMed
-
- Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36:18‐23. - PubMed
-
- Smithline HA, Mader TJ, Crenshaw BJ. Do patients with acute medical conditions have the capacity to give informed consent for emergency medicine research? Acad Emerg Med. 1999;6:776‐780. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous