Endometriosis and cardiovascular disease
- PMID: 35919664
- PMCID: PMC9242051
- DOI: 10.1093/ehjopen/oeac001
Endometriosis and cardiovascular disease
Erratum in
-
Corrigendum to articles in EHJ Open missing data availability statements.Eur Heart J Open. 2024 Jan 16;4(1):oead137. doi: 10.1093/ehjopen/oead137. eCollection 2024 Jan. Eur Heart J Open. 2024. PMID: 38230360 Free PMC article.
Abstract
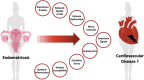
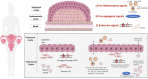
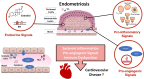
Endometriosis is a chronic gynaecological disease affecting 1 in 10 reproductive-age women. It is defined as the presence of endometrium-like tissue outside the uterus. Beyond this placid anatomical definition, endometriosis is a complex, hormonal, inflammatory, and systemic condition that poses significant familial, psychological, and economic burden. The interaction between the cardiovascular system and endometriosis has become a field of interest as the underlying mutual mechanisms become better understood. On the basis of accumulating fundamental and clinical evidence, it is likely that there exists a close relationship between endometriosis and the cardiovascular system. Therefore, investigating the endometriosis-cardiovascular interaction is highly clinically significant. In this review, we highlight our current understanding of the pathophysiology of endometriosis with systemic hormonal, pro-inflammatory, pro-angiogenic, immunologic, and genetic processes beyond the peritoneal microenvironment. Additionally, we provide current clinical evidence about how endometriosis interacts with cardiovascular risk factors and cardiovascular disease (CVD). To date, only small associations between endometriosis and CVD have been reported in observational studies, inherently limited by the potential influence of unmeasured confounding. Cardiovascular disease in women with endometriosis remains understudied, under-recognized, and underdiagnosed. More detailed study of the cardiovascular-endometriosis interaction is needed to fully understand its clinical relevance, underlying pathophysiology, possible means of early diagnosis and prevention.
Keywords: Cardiovascular disease; Endometriosis; Heart disease; Women.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

References
-
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 2018;51:1–15. - PubMed
-
- Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021;397:839–852. - PubMed
-
- Maas AHEM, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, Kunadian V, Laan E, Lambrinoudaki I, Maclaran K, Panay N, Stevenson JC, van Trotsenburg M, Collins P. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J 2021;42:967–984. - PMC - PubMed
-
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382:1244–1256. - PubMed
-
- Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017;209:3–7. - PubMed
LinkOut - more resources
Full Text Sources

