Unveiling the interplay between homogeneous and heterogeneous catalytic mechanisms in copper-iron nanoparticles working under chemically relevant tumour conditions
- PMID: 35919722
- PMCID: PMC9297535
- DOI: 10.1039/d2sc01379g
Unveiling the interplay between homogeneous and heterogeneous catalytic mechanisms in copper-iron nanoparticles working under chemically relevant tumour conditions
Abstract
The present work sheds light on a generally overlooked issue in the emerging field of bio-orthogonal catalysis within tumour microenvironments (TMEs): the interplay between homogeneous and heterogeneous catalytic processes. In most cases, previous works dealing with nanoparticle-based catalysis in the TME focus on the effects obtained (e.g. tumour cell death) and attribute the results to heterogeneous processes alone. The specific mechanisms are rarely substantiated and, furthermore, the possibility of a significant contribution of homogeneous processes by leached species - and the complexes that they may form with biomolecules - is neither contemplated nor pursued. Herein, we have designed a bimetallic catalyst nanoparticle containing Cu and Fe species and we have been able to describe the whole picture in a more complex scenario where both homogeneous and heterogeneous processes are coupled and fostered under TME relevant chemical conditions. We investigate the preferential leaching of Cu ions in the presence of a TME overexpressed biomolecule such as glutathione (GSH). We demonstrate that these homogeneous processes initiated by the released by Cu-GSH interactions are in fact responsible for the greater part of the cell death effects found (GSH, a scavenger of reactive oxygen species, is depleted and highly active superoxide anions are generated in the same catalytic cycle). The remaining solid CuFe nanoparticle becomes an active catalyst to supply oxygen from oxygen reduced species, such as superoxide anions (by-product from GSH oxidation) and hydrogen peroxide, another species that is enriched in the TME. This activity is essential to sustain the homogeneous catalytic cycle in the oxygen-deprived tumour microenvironment. The combined heterogeneous-homogeneous mechanisms revealed themselves as highly efficient in selectively killing cancer cells, due to their higher GSH levels compared to healthy cell lines.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




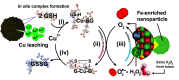
Similar articles
-
A Highly-Active Chemodynamic Agent Based on In Situ Generated Copper Complexes from Copper Hexacyanoferrate Nanoparticles.Small. 2025 Apr;21(13):e2412355. doi: 10.1002/smll.202412355. Epub 2025 Feb 21. Small. 2025. PMID: 39981844
-
Glutathione-Triggered catalytic response of Copper-Iron mixed oxide Nanoparticles. Leveraging tumor microenvironment conditions for chemodynamic therapy.J Colloid Interface Sci. 2022 Jul;617:704-717. doi: 10.1016/j.jcis.2022.03.036. Epub 2022 Mar 11. J Colloid Interface Sci. 2022. PMID: 35316784
-
Tumor microenvironment-responsive nanozymes achieve photothermal-enhanced multiple catalysis against tumor hypoxia.Acta Biomater. 2021 Nov;135:617-627. doi: 10.1016/j.actbio.2021.08.015. Epub 2021 Aug 15. Acta Biomater. 2021. PMID: 34407474
-
Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione.J Inorg Biochem. 2016 Jan;154:78-88. doi: 10.1016/j.jinorgbio.2015.08.005. Epub 2015 Aug 11. J Inorg Biochem. 2016. PMID: 26277412 Review.
-
Hydroxylamine driven advanced oxidation processes for water treatment: A review.Chemosphere. 2021 Jan;262:128390. doi: 10.1016/j.chemosphere.2020.128390. Epub 2020 Sep 21. Chemosphere. 2021. PMID: 33182154 Review.
Cited by
-
Nanoparticle-Catalyzed Transamination under Tumor Microenvironment Conditions: A Novel Tool to Disrupt the Pool of Amino Acids and GSSG in Cancer Cells.Nano Lett. 2024 Apr 10;24(14):4091-4100. doi: 10.1021/acs.nanolett.3c04947. Epub 2024 Mar 15. Nano Lett. 2024. PMID: 38489158 Free PMC article.
-
A hollow-tube-like hydrospongel for multimodal therapy of advanced colorectal cancer.Nat Commun. 2025 Aug 12;16(1):7464. doi: 10.1038/s41467-025-62880-x. Nat Commun. 2025. PMID: 40796777 Free PMC article.
-
Heterogeneous-Driven Glutathione Oxidation: Defining the Catalytic Role of Chalcopyrite Nanoparticles.J Phys Chem C Nanomater Interfaces. 2023 Jul 12;127(29):14146-14154. doi: 10.1021/acs.jpcc.3c00987. eCollection 2023 Jul 27. J Phys Chem C Nanomater Interfaces. 2023. PMID: 37529663 Free PMC article.
-
Tumor-activated in situ synthesis of single-atom catalysts for O2-independent photodynamic therapy based on water-splitting.Nat Commun. 2024 Apr 6;15(1):2954. doi: 10.1038/s41467-024-46987-1. Nat Commun. 2024. PMID: 38582750 Free PMC article.
-
Nanomedical research and development in Spain: improving the treatment of diseases from the nanoscale.Front Bioeng Biotechnol. 2023 Jul 21;11:1191327. doi: 10.3389/fbioe.2023.1191327. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37545884 Free PMC article. Review.
References
-
- Ding Y. Dai Y. Wu M. Li L. Chem. Eng. J. 2021;426:128880. doi: 10.1016/j.cej.2021.128880. - DOI
-
- Nelson D. L., Lehninger A. L. and Cox M. M., Lehninger Principles of Biochemistry, W. H. Freeman, 2008
LinkOut - more resources
Full Text Sources

