Enzyme-photo-coupled catalysis in gas-sprayed microdroplets
- PMID: 35919726
- PMCID: PMC9297532
- DOI: 10.1039/d2sc02791g
Enzyme-photo-coupled catalysis in gas-sprayed microdroplets
Abstract
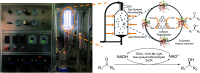
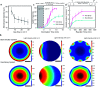
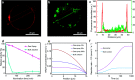
Enzyme-photo-coupled catalysis produces fine chemicals by combining the high selectivity of an enzyme with the green energy input of sunlight. Operating a large-scale system, however, remains challenging because of the significant loss of enzyme activity caused by continuous illumination and the difficulty in utilizing solar energy with high efficiency at large scale. We present a large-scale enzyme-photo-coupled catalysis system based on gas-sprayed microdroplets. By this means, we demonstrate a 43.6-71.5 times improvement of solar energy utilization over that using a traditional bulk processing system. Owing to the improved enzyme activity in microdroplets, we show that chiral alcohols can be produced with up to a 2.2-fold increase in the reaction rate and a 5.6-fold increase in final product concentration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Acceleration of Enzyme-Catalyzed Reactions at Aqueous Interfaces through Enhanced Reaction Kinetics of Microdroplets.Anal Chem. 2025 Mar 25;97(11):5992-6000. doi: 10.1021/acs.analchem.4c05595. Epub 2025 Mar 11. Anal Chem. 2025. PMID: 40067317
-
Solar oxidation of toluene over Co doped nano-catalyst.Chemosphere. 2020 Sep;255:126878. doi: 10.1016/j.chemosphere.2020.126878. Epub 2020 Apr 29. Chemosphere. 2020. PMID: 32387727
-
Sprayed Water Microdroplets Are Able to Generate Hydrogen Peroxide Spontaneously.J Am Chem Soc. 2022 May 4;144(17):7606-7609. doi: 10.1021/jacs.2c02890. Epub 2022 Apr 22. J Am Chem Soc. 2022. PMID: 35451822
-
Nanoarray Structures for Artificial Photosynthesis.Small. 2021 Sep;17(38):e2006530. doi: 10.1002/smll.202006530. Epub 2021 Apr 25. Small. 2021. PMID: 33896110 Review.
-
Fundamentals and applications of photo-thermal catalysis.Chem Soc Rev. 2021 Feb 15;50(3):2173-2210. doi: 10.1039/d0cs00357c. Chem Soc Rev. 2021. PMID: 33336654 Review.
Cited by
-
A path to perpetual chemical synthesis via photocatalytic cofactor regeneration.Chem Sci. 2025 May 23;16(25):11184-11203. doi: 10.1039/d5sc02770e. eCollection 2025 Jun 25. Chem Sci. 2025. PMID: 40453794 Free PMC article. Review.
-
Microstructured gas-liquid-(solid) interfaces: A platform for sustainable synthesis of commodity chemicals.Sci Adv. 2024 May 31;10(22):eado5448. doi: 10.1126/sciadv.ado5448. Epub 2024 May 29. Sci Adv. 2024. PMID: 38809985 Free PMC article. Review.
-
The power of microdroplet photochemistry.Chem Sci. 2024 Feb 1;15(10):3670-3672. doi: 10.1039/d4sc00056k. eCollection 2024 Mar 6. Chem Sci. 2024. PMID: 38454998 Free PMC article.
References
-
- Planavsky N. J. Asael D. Hofmann A. Reinhard C. T. Lalonde S. V. Knudsen A. Wang X. Ossa F. O. Pecoits E. Smith A. J. B. Beukes N. J. Bekker A. Johnson T. M. Konhauser K. O. Lyons T. W. Rouxel O. J. Nat. Geosci. 2014;7:283–286. doi: 10.1038/ngeo2122. - DOI
LinkOut - more resources
Full Text Sources

