Deep neural network based quantum simulations and quasichemical theory for accurate modeling of molten salt thermodynamics
- PMID: 35919729
- PMCID: PMC9297527
- DOI: 10.1039/d2sc02227c
Deep neural network based quantum simulations and quasichemical theory for accurate modeling of molten salt thermodynamics
Abstract
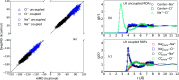
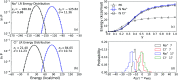
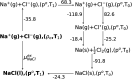
With dual goals of efficient and accurate modeling of solvation thermodynamics in molten salt liquids, we employ ab initio molecular dynamics (AIMD) simulations, deep neural network interatomic potentials (NNIP), and quasichemical theory (QCT) to calculate the excess chemical potentials for the solute ions Na+ and Cl- in the molten NaCl liquid. NNIP-based molecular dynamics simulations accelerate the calculations by 3 orders of magnitude and reduce the uncertainty to 1 kcal mol-1. Using the Density Functional Theory (DFT) level of theory, the predicted excess chemical potential for the solute ion pair is -178.5 ± 1.1 kcal mol-1. A quantum correction of 13.7 ± 1.9 kcal mol-1 is estimated via higher-level quantum chemistry calculations, leading to a final predicted ion pair excess chemical potential of -164.8 ± 2.2 kcal mol-1. The result is in good agreement with a value of -163.5 kcal mol-1 obtained from thermo-chemical tables. This study validates the application of QCT and NNIP simulations to the molten salt liquids, allowing for significant insights into the solvation thermodynamics crucial for numerous molten salt applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Liquid-Vapor Phase Equilibrium in Molten Aluminum Chloride (AlCl3) Enabled by Machine Learning Interatomic Potentials.J Phys Chem B. 2025 Jan 23;129(3):952-964. doi: 10.1021/acs.jpcb.4c06450. Epub 2025 Jan 12. J Phys Chem B. 2025. PMID: 39801049
-
Modeling LiF and FLiBe Molten Salts with Robust Neural Network Interatomic Potential.ACS Appl Mater Interfaces. 2021 Jun 2;13(21):24582-24592. doi: 10.1021/acsami.1c00604. Epub 2021 May 21. ACS Appl Mater Interfaces. 2021. PMID: 34019760
-
Exploring Cr and molten salt interfacial interactions for molten salt applications.Phys Chem Chem Phys. 2024 Aug 14;26(32):21342-21356. doi: 10.1039/d4cp01122h. Phys Chem Chem Phys. 2024. PMID: 38829308
-
Simulating Nuclear and Electronic Quantum Effects in Enzymes.Methods Enzymol. 2016;577:389-418. doi: 10.1016/bs.mie.2016.05.047. Epub 2016 Jul 15. Methods Enzymol. 2016. PMID: 27498646 Review.
-
Relationship between Solvation Thermodynamics from IST and DFT Perspectives.J Phys Chem B. 2017 Apr 20;121(15):3825-3841. doi: 10.1021/acs.jpcb.6b12889. Epub 2017 Feb 28. J Phys Chem B. 2017. PMID: 28186751 Free PMC article. Review.
Cited by
-
Computing chemical potentials with machine-learning-accelerated simulations to accurately predict thermodynamic properties of molten salts.Chem Sci. 2025 Jan 24;16(7):3078-3091. doi: 10.1039/d4sc07253g. eCollection 2025 Feb 12. Chem Sci. 2025. PMID: 39867953 Free PMC article.
References
-
- Le Brun C. J. Nucl. Mater. 2007;360:1–5. doi: 10.1016/j.jnucmat.2006.08.017. - DOI
-
- Williams D. F. Clarno K. T. Nucl. Technol. 2008;163:330–343. doi: 10.13182/NT08-A3992. - DOI
-
- Jerome S. Michel A. Ondrej B. Sylvie D. Olga F. Veronique G. Daniel H. David H. Victor I. Leen K. J. andMerle Lucotte Elsa L. L. Jan U. Ritsuo Y. Dai Z. Prog. Nucl. Energy. 2014;77:308–319. doi: 10.1016/j.pnucene.2014.02.014. - DOI
-
- Zhang H. Baeyens J. Degreve J. Caceres G. Renewable Sustainable Energy Rev. 2013;22:466–481. doi: 10.1016/j.rser.2013.01.032. - DOI
-
- Pelay U. Luo L. Fan Y. Stitou D. Rood M. Renewable Sustainable Energy Rev. 2017;79:82–100. doi: 10.1016/j.rser.2017.03.139. - DOI
LinkOut - more resources
Full Text Sources

