Upcoming immunotherapeutic combinations for B-cell lymphoma
- PMID: 35919738
- PMCID: PMC9326875
- DOI: 10.1093/immadv/ltab001
Upcoming immunotherapeutic combinations for B-cell lymphoma
Abstract
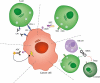
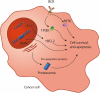
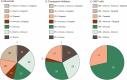
After initial introduction for B-cell lymphomas as adjuvant therapies to established cancer treatments, immune checkpoint inhibitors and other immunotherapies are now integrated in mainstream regimens, both in adult and pediatric patients. We here provide an overview of the current status of combination therapies for B-cell lymphoma, by in-depth analysis of combination therapy trials registered between 2015-2020. Our analysis provides new insight into the rapid evolution in lymphoma treatment, as propelled by new additions to the treatment arsenal. We conclude with prospects on upcoming clinical trials which will likely use systematic testing approaches of more combinations of established chemotherapy regimens with new agents, as well as new combinations of immunotherapy and targeted therapy. Future trials will be set up as basket or umbrella-type trials to facilitate the evaluation of new drugs targeting specific genetic changes in the tumor or associated immune microenvironment. As such, lymphoma patients will benefit by receiving more tailored treatment that is based on synergistic effects of chemotherapy combined with new agents targeting specific aspects of tumor biology and the immune system.
Keywords: checkpoint inhibition; hematological cancer; immunotherapy; lymphoma; tumor antigen.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures
Similar articles
-
Checkpoint Inhibitors and Other Immune Therapies for Hodgkin and Non-Hodgkin Lymphoma.Curr Treat Options Oncol. 2016 Jun;17(6):31. doi: 10.1007/s11864-016-0401-9. Curr Treat Options Oncol. 2016. PMID: 27193488 Free PMC article. Review.
-
Nouvelles perspectives dans l’immunothérapie des cancers pédiatriques.Bull Cancer. 2018 Dec;105 Suppl 1:S68-S79. doi: 10.1016/S0007-4551(18)30392-8. Bull Cancer. 2018. PMID: 30595201 Review. French.
-
Treatment of relapsed aggressive lymphomas: regimens with and without high-dose therapy and stem cell rescue.Cancer Chemother Pharmacol. 2002 May;49 Suppl 1:S13-20. doi: 10.1007/s00280-002-0447-1. Epub 2002 Apr 12. Cancer Chemother Pharmacol. 2002. PMID: 12042984 Review.
-
Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer.Drug Resist Updat. 2019 Sep;46:100644. doi: 10.1016/j.drup.2019.100644. Epub 2019 Sep 17. Drug Resist Updat. 2019. PMID: 31585395 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Toripalimab plus lenalidomide for central nervous system recurrence in refractory CD5+ diffuse large B-cell lymphoma with MYD88 and CD79B comutation: a case report.Transl Cancer Res. 2024 Feb 29;13(2):1188-1195. doi: 10.21037/tcr-23-1638. Epub 2024 Feb 23. Transl Cancer Res. 2024. PMID: 38482415 Free PMC article.
-
Emerging Immunotherapy Approaches for Treating Prostate Cancer.Int J Mol Sci. 2023 Sep 20;24(18):14347. doi: 10.3390/ijms241814347. Int J Mol Sci. 2023. PMID: 37762648 Free PMC article. Review.
-
Strategies to overcome low MHC-I expression in paediatric and adult tumours.Immunother Adv. 2023 Dec 11;4(1):ltad028. doi: 10.1093/immadv/ltad028. eCollection 2024. Immunother Adv. 2023. PMID: 38223409 Free PMC article. Review.
-
Clinical and prognostic factors guiding diagnosis and treatment of prostate diffuse large B-cell lymphoma.Sci Rep. 2025 Apr 12;15(1):12594. doi: 10.1038/s41598-025-96315-w. Sci Rep. 2025. PMID: 40221476 Free PMC article.
-
Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment.Diagnostics (Basel). 2022 Feb 3;12(2):394. doi: 10.3390/diagnostics12020394. Diagnostics (Basel). 2022. PMID: 35204484 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
