Metal-free synthesis of C2-quaternary indolinones by (NH4)2S2O8 mediated oxidative dearomatization of indoles
- PMID: 35919833
- PMCID: PMC9301541
- DOI: 10.1039/d2ra04191j
Metal-free synthesis of C2-quaternary indolinones by (NH4)2S2O8 mediated oxidative dearomatization of indoles
Abstract
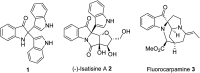
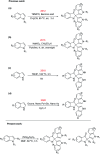
An efficient metal-free, (NH4)2S2O8 mediated oxidative dearomatization of indoles for the construction of C2-quaternary indolinones was disclosed. A series of C2-quaternary indolinones derivatives with good functional group tolerance were obtained in moderate to excellent yields. This methodology provides an alternative approach for the direct generation of all-carbon quaternary centers at the C2 position of indoles. This catalytic approach represents a step-economic and convenient strategy for the oxidative dearomatization of indoles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Metal-free catalyzed oxidative trimerization of indoles by using TEMPO in air: a biomimetic approach to 2-(1H-indol-3-yl)-2,3'-biindolin-3-ones.Org Biomol Chem. 2012 Nov 28;10(44):8814-21. doi: 10.1039/c2ob26390d. Org Biomol Chem. 2012. PMID: 23044781
-
(NH4)2S2O8-Mediated Metal-Free Decarboxylative Formylation/Acylation of α-Oxo/Ketoacids and Its Application to the Synthesis of Indole Alkaloids.J Org Chem. 2022 Aug 5;87(15):10359-10365. doi: 10.1021/acs.joc.2c00552. Epub 2022 Jul 12. J Org Chem. 2022. PMID: 35820161
-
Divergent Synthesis of Indolenine and Indoline Ring Systems by Palladium-Catalyzed Asymmetric Dearomatization of Indoles.Angew Chem Int Ed Engl. 2022 Mar 21;61(13):e202116024. doi: 10.1002/anie.202116024. Epub 2022 Feb 9. Angew Chem Int Ed Engl. 2022. PMID: 35080106
-
Asymmetric Dearomatization of Indole Derivatives with N-Hydroxycarbamates Enabled by Photoredox Catalysis.Angew Chem Int Ed Engl. 2019 Dec 9;58(50):18069-18074. doi: 10.1002/anie.201911144. Epub 2019 Oct 24. Angew Chem Int Ed Engl. 2019. PMID: 31587423 Review.
-
Application of Metal-Free Dearomatization Reaction as a Sustainable Strategy to Direct Access Complex Cyclic Compounds.Chem Rec. 2023 Oct;23(10):e202300101. doi: 10.1002/tcr.202300101. Epub 2023 May 2. Chem Rec. 2023. PMID: 37132130 Review.
References
-
- Vitaku E. Smith D. T. Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 2014;57:10257–10274. - PubMed
- Taylor R. D. MacCoss M. Lawson A. D. Rings in drugs: Miniperspective. J. Med. Chem. 2014;57:5845–5859. - PubMed
-
- Sundberg R., Indoles, Academic Press, San Diego, 1996, p. 113
- Cacchi S. Fabrizi G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005;105:2873–2920. - PubMed
- Joucla L. Djakovitch L. Transition Metal-Catalysed, Direct and Site-Selective N1-, C2-or C3-Arylation of the Indole Nucleus: 20 Years of Improvements. Adv. Synth. Catal. 2009;351:673–714.
- Bandini M. Eichholzer A. Catalytic functionalization of indoles in a new dimension. Angew. Chem., Int. Ed. 2009;48:9608–9644. - PubMed
- Bartoli G. Bencivenni G. Dalpozzo R. Organocatalytic strategies for the asymmetric functionalization of indoles. Chem. Soc. Rev. 2010;39:4449–4465. - PubMed
-
- Gazit A. Osherov N. Posner I. Yaish P. Poradosu E. Gilon C. Levitzki A. Tyrphostins. II. Heterocyclic and. alpha.-substituted benzylidenemalononitrile tyrphostins as potent inhibitors of EGF receptor and ErbB2/neu tyrosine kinases. J. Med. Chem. 1991;34:1896–1907. - PubMed
- Dilber S. Saban M. Jelaca J. Gelineo A. Arsenijević L. Bogavac M. Investigation of antimicrobial activity of some isatin derivatives. Pharmazie. 1989;44:649–650. - PubMed
- Wetzel A. Gagosz F. Gold-Catalyzed Transformation of 2-Alkynyl Arylazides: Efficient Access to the Valuable Pseudoindoxyl and Indolyl Frameworks. Angew. Chem., Int. Ed. 2011;123:7492–7496. - PubMed
- Kumar C. V. S. Puranik V. G. Ramana C. V. InCl3-Mediated Addition of Indole to Isatogens: An Expeditious Synthesis of 13-deoxy-Isatisine A. Chem.–Eur. J. 2012;18:9601–9611. - PubMed
-
- Linhares M. Rebelo S. L. Simoes M. M. Silva A. M. Neves M. G. P. Cavaleiro J. A. Freire C. Biomimetic oxidation of indole by Mn (III) porphyrins. Appl. Catal. 2014;470:427–433.
-
- Bell R. Carmeli S. Sar N. Vibrindole A, a metabolite of the marine bacterium, Vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish Ostracion cubicus. J. Nat. Prod. 1994;57:1587–1590. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous