Eumelanin pigment precursor 2-carboxy-5,6-dihydroxyindole and 2-amino-6-methylbenzothiazole chromophore integration towards melanin inspired chemoresponsive materials: the case of the Zn2+ ion
- PMID: 35919835
- PMCID: PMC9301554
- DOI: 10.1039/d2ra02616c
Eumelanin pigment precursor 2-carboxy-5,6-dihydroxyindole and 2-amino-6-methylbenzothiazole chromophore integration towards melanin inspired chemoresponsive materials: the case of the Zn2+ ion
Abstract
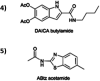
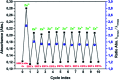
The 2-amino-6-methylbenzothiazole chromophore is introduced at the carboxyl group of the melanin precursor 2-carboxy-5,6-dihydroxyindole achieving a novel dihydroxyindole derivative with metal chelation properties not depending on the catechol moiety. In view of potential exploitation in charge storage systems, systematic investigation of the interaction of the new amide derivative with metal ions is carried out, in comparison with that of the parent 2-carboxy-5,6-dihydroxyindole, and the stoichiometry of the zinc-amide complex is determined.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




Similar articles
-
Eumelanin Precursor 2-Carboxy-5,6-Dihydroxyindole (DHICA) as Doping Factor in Ternary (PEDOT:PSS/Eumelanin) Thin Films for Conductivity Enhancement.Materials (Basel). 2020 May 2;13(9):2108. doi: 10.3390/ma13092108. Materials (Basel). 2020. PMID: 32370189 Free PMC article.
-
Degree of polymerization of 5,6-dihydroxyindole-derived eumelanin from chemical degradation study.Pigment Cell Melanoma Res. 2014 Jul;27(4):664-7. doi: 10.1111/pcmr.12254. Epub 2014 May 12. Pigment Cell Melanoma Res. 2014. PMID: 24750564
-
Metal binding by melanins: studies of colloidal dihydroxyindole-melanin, and its complexation by Cu(II) and Zn(II) ions.J Inorg Biochem. 2002 Apr 10;89(1-2):45-53. doi: 10.1016/s0162-0134(01)00406-8. J Inorg Biochem. 2002. PMID: 11931962
-
Current update and trends in melanin pigmentation and melanin biology.Keio J Med. 1995 Mar;44(1):9-18. doi: 10.2302/kjm.44.9. Keio J Med. 1995. PMID: 7760535 Review.
-
Reactivities of Quinone Methides versus o-Quinones in Catecholamine Metabolism and Eumelanin Biosynthesis.Int J Mol Sci. 2016 Sep 20;17(9):1576. doi: 10.3390/ijms17091576. Int J Mol Sci. 2016. PMID: 27657049 Free PMC article. Review.
References
-
- Stavila V. Talin A. A. Allendorf M. D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014;43(16):5994–6010. - PubMed
-
- Fuentes N. Martin-Lasanta A. Alvarez de Cienfuegos L. Ribagorda M. Parra A. Cuerva J. M. Organic-based molecular switches for molecular electronics. Nanoscale. 2011;3(10):4003–4014. - PubMed
-
- Leith G. A. Martin C. R. Mathur A. Kittikhunnatham P. Park K. C. Shustova N. B. Dynamically Controlled Electronic Behavior of Stimuli-Responsive Materials: Exploring Dimensionality and Connectivity. Adv. Energy Mater. 2022;12(4):2100441.
-
- Seeboth A. Lotzsch D. Ruhmann R. Muehling O. Thermochromic polymers–function by design. Chem. Rev. 2014;114(5):3037–3068. - PubMed
LinkOut - more resources
Full Text Sources

