Photocatalytic Reduction of Nicotinamide Co-factor by Perylene Sensitized RhIII Complexes
- PMID: 35920047
- PMCID: PMC9825842
- DOI: 10.1002/chem.202201931
Photocatalytic Reduction of Nicotinamide Co-factor by Perylene Sensitized RhIII Complexes
Abstract
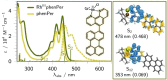
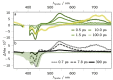
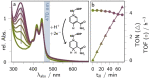
The ambitious goal of artificial photosynthesis is to develop active systems that mimic nature and use light to split water into hydrogen and oxygen. Intramolecular design concepts are particularly promising. Herein, we firstly present an intramolecular photocatalyst integrating a perylene-based light-harvesting moiety and a catalytic rhodium center (RhIII phenPer). The excited-state dynamics were investigated by means of steady-state and time-resolved absorption and emission spectroscopy. The studies reveal that photoexcitation of RhIII phenPer yields the formation of a charge-separated intermediate, namely RhII phenPer⋅+ , that results in a catalytically active species in the presence of protons.
Keywords: charge separation; perylene; photocatalysis; rhodium catalyst; time-resolved spectroscopy.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water.Molecules. 2022 Oct 5;27(19):6614. doi: 10.3390/molecules27196614. Molecules. 2022. PMID: 36235152 Free PMC article.
-
Unraveling the Light-Activated Reaction Mechanism in a Catalytically Competent Key Intermediate of a Multifunctional Molecular Catalyst for Artificial Photosynthesis.Angew Chem Int Ed Engl. 2019 Sep 9;58(37):13140-13148. doi: 10.1002/anie.201907247. Epub 2019 Aug 19. Angew Chem Int Ed Engl. 2019. PMID: 31347251 Free PMC article. Review.
-
Denaturation of Lysozyme with Visible-light-responsive Photocatalysts of Ground Rhodium-doped and Ground Rhodium-antimony-co-doped Strontium Titanate.J Oleo Sci. 2018;67(12):1521-1533. doi: 10.5650/jos.ess18155. J Oleo Sci. 2018. PMID: 30504623
-
Evaluation of C4 diphosphine ligands in rhodium catalysed methanol carbonylation under a syngas atmosphere: synthesis, structure, stability and reactivity of rhodium(I) carbonyl and rhodium(III) acetyl intermediates.Dalton Trans. 2007 Dec 21;(47):5582-9. doi: 10.1039/b712974b. Epub 2007 Oct 10. Dalton Trans. 2007. PMID: 18043821
-
Emission Spectroscopy as a Probe into Photoinduced Intramolecular Electron Transfer in Polyazine Bridged Ru(II),Rh(III) Supramolecular Complexes.Materials (Basel). 2010 Aug 11;3(8):4328-4354. doi: 10.3390/ma3084328. Materials (Basel). 2010. PMID: 28883332 Free PMC article. Review.
Cited by
-
Triplet quenching pathway control with molecular dyads enables the identification of a highly oxidizing annihilator class.Chem Sci. 2023 Jul 17;14(32):8583-8591. doi: 10.1039/d3sc01725g. eCollection 2023 Aug 16. Chem Sci. 2023. PMID: 37592982 Free PMC article.
References
-
- Kranz C., Wächtler M., Chem. Soc. Rev. 2021, 50, 1407–1437. - PubMed
-
- Esswein A. J., Nocera D. G., Chem. Rev. 2007, 107, 4022–4047. - PubMed
-
- Knör G., Coord. Chem. Rev. 2015, 304–305, 102–108.
-
- Roncali J., Chem. Rev. 1992, 92, 711–738.
-
- Mishra A., Ma C.-Q., Bäuerle P., Chem. Rev. 2009, 109, 1141–1276. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

