Proton Transfer Pathways in Nitrogenase with and without Dissociated S2B
- PMID: 35920055
- PMCID: PMC9804283
- DOI: 10.1002/anie.202208544
Proton Transfer Pathways in Nitrogenase with and without Dissociated S2B
Abstract
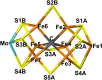
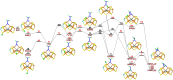
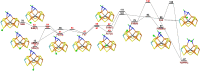
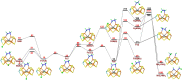
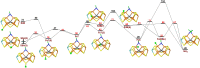
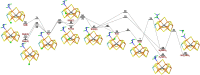
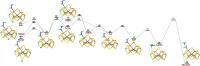
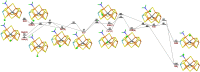
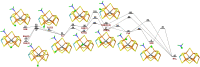
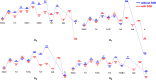
Nitrogenase is the only enzyme that can convert N2 to NH3 . Crystallographic structures have indicated that one of the sulfide ligands of the active-site FeMo cluster, S2B, can be replaced by an inhibitor, like CO and OH- , and it has been suggested that it may be displaced also during the normal reaction. We have investigated possible proton transfer pathways within the FeMo cluster during the conversion of N2 H2 to two molecules of NH3 , assuming that the protons enter the cluster at the S3B, S4B or S5A sulfide ions and are then transferred to the substrate. We use combined quantum mechanical and molecular mechanical (QM/MM) calculations with the TPSS and B3LYP functionals. The calculations indicate that the barriers for these reactions are reasonable if the S2B ligand remains bound to the cluster, but they become prohibitively high if S2B has dissociated. This suggests that it is unlikely that S2B reversibly dissociates during the normal reaction cycle.
Keywords: QM/MM; S2B dissociation; nitrogenase; proton transfer; reaction mechanisms.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
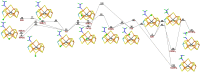
Similar articles
-
Putative reaction mechanism of nitrogenase with a half-dissociated S2B ligand.Dalton Trans. 2024 Jul 9;53(27):11500-11513. doi: 10.1039/d4dt00937a. Dalton Trans. 2024. PMID: 38916132
-
How feasible is the reversible S-dissociation mechanism for the activation of FeMo-co, the catalytic site of nitrogenase?Dalton Trans. 2019 Jan 28;48(4):1251-1262. doi: 10.1039/c8dt04531c. Epub 2019 Jan 4. Dalton Trans. 2019. PMID: 30607401
-
Thermodynamically Favourable States in the Reaction of Nitrogenase without Dissociation of any Sulfide Ligand.Chemistry. 2022 Mar 7;28(14):e202103933. doi: 10.1002/chem.202103933. Epub 2022 Feb 2. Chemistry. 2022. PMID: 35006641 Free PMC article.
-
The nitrogenase mechanism: new roles for the dangler?J Biol Inorg Chem. 2025 Mar;30(2):125-133. doi: 10.1007/s00775-024-02085-7. Epub 2024 Dec 19. J Biol Inorg Chem. 2025. PMID: 39699648 Free PMC article. Review.
-
Computational Investigations of the Chemical Mechanism of the Enzyme Nitrogenase.Chembiochem. 2020 Jun 15;21(12):1671-1709. doi: 10.1002/cbic.201900636. Epub 2020 Jan 21. Chembiochem. 2020. PMID: 31803989 Review.
Cited by
-
Biological nitrogen fixation in theory, practice, and reality: a perspective on the molybdenum nitrogenase system.FEBS Lett. 2023 Jan;597(1):45-58. doi: 10.1002/1873-3468.14534. Epub 2022 Nov 28. FEBS Lett. 2023. PMID: 36344435 Free PMC article. Review.
-
Final E5 to E8 Steps in the Nitrogenase Mechanism for Nitrogen Fixation.J Phys Chem B. 2024 Oct 10;128(40):9699-9705. doi: 10.1021/acs.jpcb.4c04331. Epub 2024 Sep 30. J Phys Chem B. 2024. PMID: 39344806 Free PMC article.
-
Cooperative Sulfur Transformations at a Dinickel Site: A Metal Bridging Sulfur Radical and Its H-Atom Abstraction Thermochemistry.J Am Chem Soc. 2024 Aug 21;146(33):23158-23170. doi: 10.1021/jacs.4c05113. Epub 2024 Aug 7. J Am Chem Soc. 2024. PMID: 39110481 Free PMC article.
-
Reaction Mechanism for CO Reduction by Mo-Nitrogenase Studied by QM/MM.Inorg Chem. 2024 Aug 26;63(34):15951-15963. doi: 10.1021/acs.inorgchem.4c02323. Epub 2024 Aug 14. Inorg Chem. 2024. PMID: 39141025 Free PMC article.
-
Quantum Mechanical Calculations of Redox Potentials of the Metal Clusters in Nitrogenase.Molecules. 2022 Dec 21;28(1):65. doi: 10.3390/molecules28010065. Molecules. 2022. PMID: 36615260 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

