LncRNA GAS5 promotes spermidine‑induced autophagy through the miRNA‑31‑5p/NAT8L axis in pulmonary artery endothelial cells of patients with CTEPH
- PMID: 35920180
- PMCID: PMC9434988
- DOI: 10.3892/mmr.2022.12813
LncRNA GAS5 promotes spermidine‑induced autophagy through the miRNA‑31‑5p/NAT8L axis in pulmonary artery endothelial cells of patients with CTEPH
Abstract
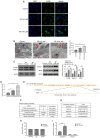
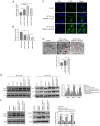
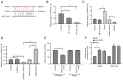
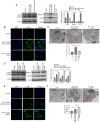
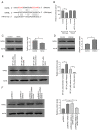
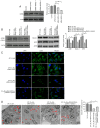
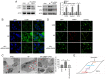
Chronic thromboembolic pulmonary hypertension (CTEPH) is a leading cause of pulmonary hypertension. The present study investigated the mechanisms of long non‑coding RNA growth arrest‑specific transcript 5 (GAS5) on spermidine (SP)‑induced autophagy. Pulmonary artery endothelial cells (PAECs) were collected from patients with CTEPH and the rat model. Immunofluorescence, Western blots, reverse transcription‑quantitative polymerase chain reaction, bioinformatics, rapid amplification of cDNA ends assays, luciferase reporter assays, RNA‑binding protein immunoprecipitation assays, GFP‑LC3 adenoviruses, tfLC3 assays and transmission electron microscopy were performed. The results revealed that SP‑induced autophagy increased GAS5 in PAECs. The upregulation of GAS5 enhanced and the downregulation of GAS5 reversed the roles of SP in PAECs. Furthermore, GAS5 promoted SP‑induced autophagy in PAECs by targeting miRNA‑31‑5p. The miRNA‑31‑5p mimic suppressed and the inhibitor promoted SP‑induced autophagy. Furthermore, N‑Acetyltransferase 8 Like (NAT8L) was a target gene of miRNA‑31‑5p and knockdown of NAT8L inhibited the autophagic levels of PAECs. In vivo, SP treatment decreased miRNA‑31‑5p and increased NAT8L levels, which was reversed by the knockdown of GAS5. The downregulation of GAS5 abolished the stimulatory role of SP in PAECs of CTEPH rats. In conclusion, GAS5 promoted SP‑induced autophagy through miRNA‑31‑5p/NAT8L signaling pathways in vitro and in vivo and GAS5 may be a promising molecular marker for therapies of CTEPH.1.
Keywords: N‑Acetyltransferase 8 Like; autophagy; chronic thromboembolic pulmonary hypertension; lncRNA growth arrest‑specific transcript 5; miRNA‑31‑5p; pulmonary artery endothelial cell; spermidine.
Conflict of interest statement
The authors declare that they have no competining interests.
Figures

Similar articles
-
LncRNA-GAS5/miR-382-3p axis inhibits pulmonary artery remodeling and promotes autophagy in chronic thromboembolic pulmonary hypertension.Genes Genomics. 2022 Apr;44(4):395-404. doi: 10.1007/s13258-021-01202-z. Epub 2022 Jan 23. Genes Genomics. 2022. PMID: 35066809
-
Long‑chain non‑coding RNA GAS5 promotes cell autophagy by modulating the miR‑181c‑5p/ATG5 and miR‑1192/ATG12 axes.Int J Mol Med. 2021 Dec;48(6):209. doi: 10.3892/ijmm.2021.5042. Epub 2021 Oct 5. Int J Mol Med. 2021. PMID: 34608496 Free PMC article.
-
Tissue factor regulates autophagy in pulmonary artery endothelial cells from chronic thromboembolic pulmonary hypertension rats via the p38 MAPK-FoxO1 pathway.Respir Res. 2024 Jun 28;25(1):261. doi: 10.1186/s12931-024-02886-z. Respir Res. 2024. PMID: 38943142 Free PMC article.
-
Effect of the LncRNA GAS5-MiR-23a-ATG3 Axis in Regulating Autophagy in Patients with Breast Cancer.Cell Physiol Biochem. 2018;48(1):194-207. doi: 10.1159/000491718. Epub 2018 Jul 13. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2020;54(5):1096. doi: 10.33594/000000298. PMID: 30007957 Retracted.
-
lncR-GAS5 upregulates the splicing factor SRSF10 to impair endothelial autophagy, leading to atherogenesis.Front Med. 2023 Apr;17(2):317-329. doi: 10.1007/s11684-022-0931-4. Epub 2023 Jan 16. Front Med. 2023. PMID: 36645633
Cited by
-
Roles of LncRNAs in the Pathogenesis of Pulmonary Hypertension.Rev Cardiovasc Med. 2024 Jun 17;25(6):217. doi: 10.31083/j.rcm2506217. eCollection 2024 Jun. Rev Cardiovasc Med. 2024. PMID: 39076325 Free PMC article. Review.
-
Fetal hypoplastic lungs have multilineage inflammation that is reversed by amniotic fluid stem cell extracellular vesicle treatment.Sci Adv. 2024 Jul 26;10(30):eadn5405. doi: 10.1126/sciadv.adn5405. Epub 2024 Jul 26. Sci Adv. 2024. PMID: 39058789 Free PMC article.
-
Emerging Epigenetic Targets and Their Molecular Impact on Vascular Remodeling in Pulmonary Hypertension.Cells. 2024 Jan 28;13(3):244. doi: 10.3390/cells13030244. Cells. 2024. PMID: 38334636 Free PMC article. Review.
-
Emerging connectivity of programmed cell death pathways and pulmonary vascular remodelling during pulmonary hypertension.J Cell Mol Med. 2024 Aug;28(16):e70003. doi: 10.1111/jcmm.70003. J Cell Mol Med. 2024. PMID: 39153207 Free PMC article. Review.
-
miRNAs in Pulmonary Hypertension: Mechanistic Insights and Therapeutic Potential.Biomedicines. 2025 Aug 5;13(8):1910. doi: 10.3390/biomedicines13081910. Biomedicines. 2025. PMID: 40868164 Free PMC article. Review.
References
-
- Sakao S, Tatsumi K. Crosstalk between endothelial cell and thrombus in chronic thromboembolic pulmonary hypertension: Perspective. Histol Histopathol. 2013;28:185–193. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

