Passive Immunization with a Single Monoclonal Neutralizing Antibody Protects against Cutaneous and Mucosal Mouse Papillomavirus Infections
- PMID: 35920658
- PMCID: PMC9400481
- DOI: 10.1128/jvi.00703-22
Passive Immunization with a Single Monoclonal Neutralizing Antibody Protects against Cutaneous and Mucosal Mouse Papillomavirus Infections
Abstract
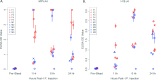
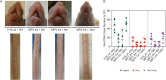
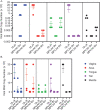
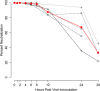
We have established a mouse papillomavirus (MmuPV1) model that induces both cutaneous and mucosal infections and cancers. In the current study, we use this model to test our hypothesis that passive immunization using a single neutralizing monoclonal antibody can protect both cutaneous and mucosal sites at different time points after viral inoculation. We conducted a series of experiments involving the administration of either a neutralizing monoclonal antibody, MPV.A4, or control monoclonal antibodies to both outbred and inbred athymic mice. Three clinically relevant mucosal sites (lower genital tract for females and anus and tongue for both males and females) and two cutaneous sites (muzzle and tail) were tested. At the termination of the experiments, all tested tissues were harvested for virological analyses. Significantly lower levels of viral signals were detected in the MPV.A4-treated female mice up to 6 h post-viral inoculation compared to those in the isotype control. Interestingly, males displayed partial protection when they received MPV.A4 at the time of viral inoculation, even though they were completely protected when receiving MPV.A4 at 24 h before viral inoculation. We detected MPV.A4 in the blood starting at 1 h and up to 8 weeks postadministration in some mice. Parallel to these in vivo studies, we conducted in vitro neutralization using a mouse keratinocyte cell line and observed complete neutralization up to 8 h post-viral inoculation. Thus, passive immunization with a monoclonal neutralizing antibody can protect against papillomavirus infection at both cutaneous and mucosal sites and is time dependent. IMPORTANCE This is the first study testing a single monoclonal neutralizing antibody (MPV.A4) by passive immunization against papillomavirus infections at both cutaneous and mucosal sites in the same host in the mouse papillomavirus model. We demonstrated that MPV.A4 administered before viral inoculation can protect both male and female athymic mice against MmuPV1 infections at cutaneous and mucosal sites. MPV.A4 also offers partial protection at 6 h post-viral inoculation in female mice. MPV.A4 can be detected in the blood from 1 h to 8 weeks after intraperitoneal (i.p.) injection. Interestingly, males were only partially protected when they received MPV.A4 at the time of viral inoculation. The failed protection in males was due to the absence of neutralizing MPV.A4 at the infected sites. Our findings suggest passive immunization with a single monoclonal neutralizing antibody can protect against diverse papillomavirus infections in a time-dependent manner in mice.
Keywords: anogenital tract; cutaneous; cutaneous infection; in situ analysis; in vivo; monoclonal antibody; mouse papillomavirus (MmuPV1); mucosal; mucosal infections; neutralizing; oral cavity; papillomavirus; passive immunization; sex difference; sexually transmitted diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus.J Virol. 2018 Jul 17;92(15):e00572-18. doi: 10.1128/JVI.00572-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743371 Free PMC article.
-
Mouse papillomavirus infection persists in mucosal tissues of an immunocompetent mouse strain and progresses to cancer.Sci Rep. 2017 Dec 5;7(1):16932. doi: 10.1038/s41598-017-17089-4. Sci Rep. 2017. PMID: 29208932 Free PMC article.
-
Mouse papillomavirus infections spread to cutaneous sites with progression to malignancy.J Gen Virol. 2017 Oct;98(10):2520-2529. doi: 10.1099/jgv.0.000926. J Gen Virol. 2017. PMID: 28942760 Free PMC article.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Papillomavirus-like particle vaccines.J Natl Cancer Inst Monogr. 2001;(28):50-4. doi: 10.1093/oxfordjournals.jncimonographs.a024258. J Natl Cancer Inst Monogr. 2001. PMID: 11158207 Review.
Cited by
-
Monitoring mouse papillomavirus-associated cancer development using longitudinal Pap smear screening.mBio. 2024 Aug 14;15(8):e0142024. doi: 10.1128/mbio.01420-24. Epub 2024 Jul 16. mBio. 2024. PMID: 39012151 Free PMC article.
-
Immune Responses in Oral Papillomavirus Clearance in the MmuPV1 Mouse Model.Pathogens. 2023 Dec 14;12(12):1452. doi: 10.3390/pathogens12121452. Pathogens. 2023. PMID: 38133335 Free PMC article.
-
Intraperitoneal delivery of cannabidiol (CBD) and Δ9-tetrahydocannabinol (THC) promotes papillomavirus infections in athymic nude mice.Tumour Virus Res. 2025 Jun;19:200307. doi: 10.1016/j.tvr.2024.200307. Epub 2024 Dec 16. Tumour Virus Res. 2025. PMID: 39694192 Free PMC article.
-
FLT3L governs the development of partially overlapping hematopoietic lineages in humans and mice.Cell. 2024 May 23;187(11):2817-2837.e31. doi: 10.1016/j.cell.2024.04.009. Epub 2024 May 3. Cell. 2024. PMID: 38701783 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

