New-onset IgA nephropathy following COVID-19 vaccination
- PMID: 35920797
- PMCID: PMC9450102
- DOI: 10.1093/qjmed/hcac185
New-onset IgA nephropathy following COVID-19 vaccination
Erratum in
-
Correction to: New-onset IgA nephropathy following COVID-19 vaccination.QJM. 2023 Feb 14;116(1):93. doi: 10.1093/qjmed/hcac239. QJM. 2023. PMID: 36272415 Free PMC article. No abstract available.
Abstract
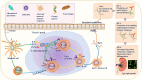
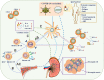
Coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused significant economic and health damage worldwide. Rapid vaccination is one of the key strategies to curb severe illness and death due to SARS-CoV-2 infection. Hundreds of millions of people worldwide have received various COVID-19 vaccines, including mRNA vaccines, inactivated vaccines and adenovirus-vectored vaccines, but the side effects and efficacy of most vaccines have not been extensively studied. Recently, there have been increasing reports of immunoglobulin A nephropathy (IgAN) after COVID-19 vaccination, however, whether their relationship is causal or coincidental remains to be verified. Here, we summarize the latest clinical evidence of IgAN diagnosed by renal biopsy associated with the COVID-19 vaccine published by 10 July 2022 with the largest sample size, and propose a hypothesis for the pathogenesis between them. At the same time, the new opportunity presented by COVID-19 vaccine allows us to explore the mechanism of IgAN recurrence for the first time. Indeed, we recognize that large-scale COVID-19 vaccination has enormous benefits in preventing COVID-19 morbidity and mortality. The purpose of this review is to help guide the clinical assessment and management of IgA nephropathy post-COVID-19 vaccination and to enrich the 'multi-hit' theory of IgA nephropathy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Association of Physicians.
Figures
Similar articles
-
Sibling cases of gross hematuria and newly diagnosed IgA nephropathy following SARS-CoV-2 vaccination.BMC Nephrol. 2022 Jun 21;23(1):216. doi: 10.1186/s12882-022-02843-2. BMC Nephrol. 2022. PMID: 35729514 Free PMC article.
-
COVID-19 mRNA vaccination is associated with IgA nephropathy: an analysis of the Japanese adverse drug event report database.J Pharm Pharm Sci. 2023 Jun 30;26:11453. doi: 10.3389/jpps.2023.11453. eCollection 2023. J Pharm Pharm Sci. 2023. PMID: 37456806 Free PMC article.
-
Secondary immunoglobulin A nephropathy with gross hematuria leading to rapidly progressive glomerulonephritis following severe acute respiratory syndrome coronavirus 2 vaccination: a case report.BMC Nephrol. 2023 Aug 8;24(1):232. doi: 10.1186/s12882-023-03287-y. BMC Nephrol. 2023. PMID: 37553599 Free PMC article.
-
New-onset autoimmune phenomena post-COVID-19 vaccination.Immunology. 2022 Apr;165(4):386-401. doi: 10.1111/imm.13443. Epub 2022 Jan 7. Immunology. 2022. PMID: 34957554 Review.
-
New insights into the mucosal immune pathogenesis of IgA nephropathy from the perspective of COVID-19 vaccination.QJM. 2023 Mar 27;116(3):181-195. doi: 10.1093/qjmed/hcac287. QJM. 2023. PMID: 36592052 Review.
Cited by
-
Clinical outcomes of Omicron infection and vaccine acceptance among pediatric liver transplant recipients: insights from a cross-sectional survey.Virol J. 2024 Nov 22;21(1):299. doi: 10.1186/s12985-024-02531-7. Virol J. 2024. PMID: 39578871 Free PMC article.
-
Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases.J Taibah Univ Med Sci. 2023 Sep 5;18(6):1646-1661. doi: 10.1016/j.jtumed.2023.08.004. eCollection 2023 Dec. J Taibah Univ Med Sci. 2023. PMID: 37732332 Free PMC article. Review.
-
An adolescent presenting with IgA nephropathy and persistent decreased kidney function after COVID-19 vaccination during follow-up for asymptomatic hematuria: a clinicopathological study.CEN Case Rep. 2025 Aug;14(4):635-640. doi: 10.1007/s13730-025-00989-0. Epub 2025 Apr 13. CEN Case Rep. 2025. PMID: 40221577 Free PMC article.
-
New-Onset and Relapsed Membranous Nephropathy post SARS-CoV-2 and COVID-19 Vaccination.Viruses. 2022 Sep 28;14(10):2143. doi: 10.3390/v14102143. Viruses. 2022. PMID: 36298697 Free PMC article. Review.
-
Safety and effectiveness of COVID-19 vaccines in patients with IgA nephropathy: a retrospective cohort study from the TriNetX global collaborative networks.EClinicalMedicine. 2023 Nov 3;65:102306. doi: 10.1016/j.eclinm.2023.102306. eCollection 2023 Nov. EClinicalMedicine. 2023. PMID: 38021374 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

