Tuning Cobalt(II) Phosphine Complexes to be Axially Ambivalent
- PMID: 35920800
- PMCID: PMC9387527
- DOI: 10.1021/acs.inorgchem.2c01562
Tuning Cobalt(II) Phosphine Complexes to be Axially Ambivalent
Abstract
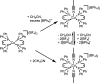
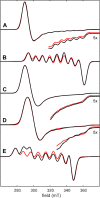
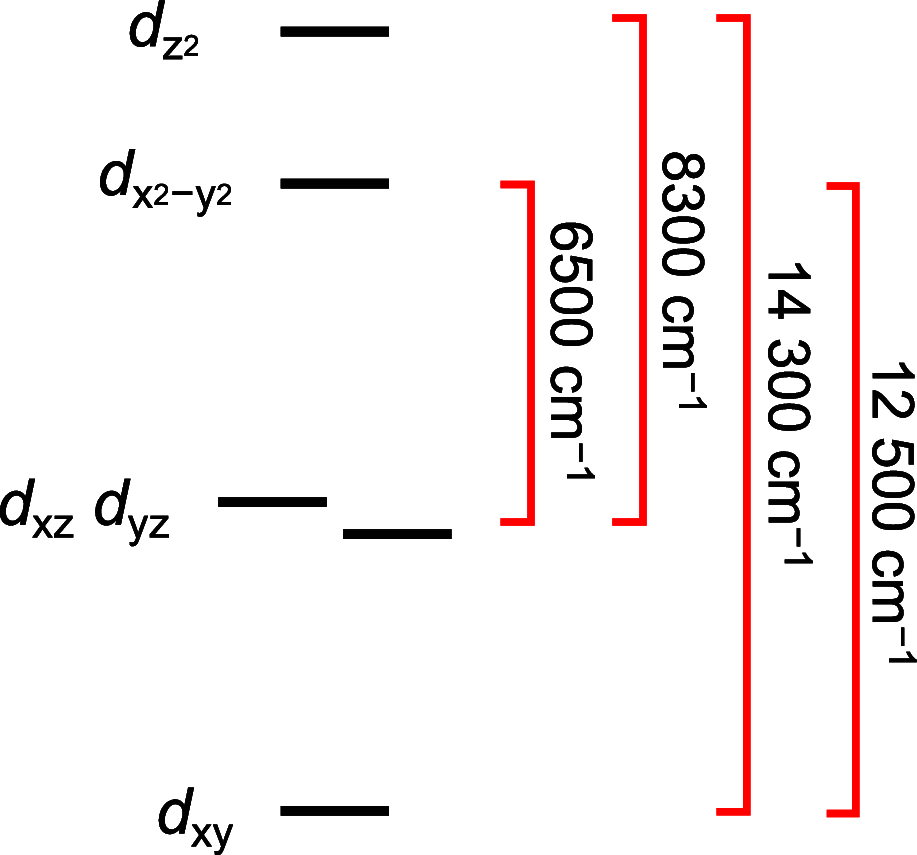
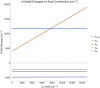
We report the isolation and characterization of a series of three cobalt(II) bis(phosphine) complexes with varying numbers of coordinated solvent ligands in the axial position. X-ray quality crystals of [Co(dppv)2][BF4]2 (1), [Co(dppv)2(NCCH3)][BPh4]2 (2), and [Co(dppv)2(NCCH3)2][BF4]2 (3) (dppv = cis-1,2-bis(diphenylphosphino)ethylene) were grown under slightly different conditions, and their structures were compared. This analysis revealed multiple crystallization motifs for divalent cobalt(II) complexes with the same set of phosphine ligands. Notably, the 4-coordinate complex 1 is a rare example of a square-planar cobalt(II) complex, the first crystallographically characterized square-planar Co(II) complex containing only neutral, bidentate ligands. Characterization of the different axial geometries via EPR and UV-visible spectroscopies showed that there is a very shallow energy landscape for axial ligation. Ligand field angular overlap model calculations support this conclusion, and we provide a strategy for tuning other ligands to be axially labile on a phosphine scaffold. This methodology is proposed to be used for designing cobalt phosphine catalysts for a variety of oxidation and reduction reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Haynes W. M.; Lide D. R.; Bruno T. J.. CRC Handbook of Chemistry and Physics; CRC press, 2016.
-
- Kurtz D. A.; Dhar D.; Elgrishi N.; Kandemir B.; McWilliams S. F.; Howland W. C.; Chen C.-H.; Dempsey J. L. Redox-Induced Structural Reorganization Dictates Kinetics of Cobalt(III) Hydride Formation via Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2021, 143, 3393–3406. 10.1021/jacs.0c11992. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

