Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Müller cells
- PMID: 35920844
- PMCID: PMC9630214
- DOI: 10.1007/s00125-022-05775-6
Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Müller cells
Abstract
Aims/hypothesis: CD40 expressed in Müller cells is a central driver of diabetic retinopathy. CD40 causes phospholipase Cγ1 (PLCγ1)-dependent ATP release in Müller cells followed by purinergic receptor (P2X7)-dependent production of proinflammatory cytokines in myeloid cells. In the diabetic retina, CD40 and P2X7 upregulate a broad range of inflammatory molecules that promote development of diabetic retinopathy. The molecular event downstream of CD40 that activates the PLCγ1-ATP-P2X7-proinflammatory cytokine cascade and promotes development of diabetic retinopathy is unknown. We hypothesise that disruption of the CD40-driven molecular events that trigger this cascade prevents/treats diabetic retinopathy in mice.
Methods: B6 and transgenic mice with Müller cell-restricted expression of wild-type (WT) CD40 or CD40 with mutations in TNF receptor-associated factor (TRAF) binding sites were made diabetic using streptozotocin. Leucostasis was assessed using FITC-conjugated concanavalin A. Histopathology was examined in the retinal vasculature. Expression of inflammatory molecules and phospho-Tyr783 PLCγ1 (p-PLCγ1) were assessed using real-time PCR, immunoblot and/or immunohistochemistry. Release of ATP and cytokines were measured by ATP bioluminescence and ELISA, respectively.
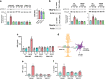
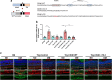
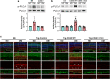
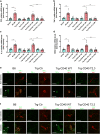
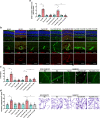
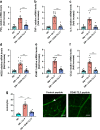
Results: Human Müller cells with CD40 ΔT2,3 (lacks TRAF2,3 binding sites) were unable to phosphorylate PLCγ1 and release ATP in response to CD40 ligation, and could not induce TNF-α/IL-1β secretion in bystander myeloid cells. CD40-TRAF signalling acted via Src to induce PLCγ1 phosphorylation. Diabetic mice in which WT CD40 in Müller cells was replaced by CD40 ΔT2,3 failed to exhibit phosphorylation of PLCγ1 in these cells and upregulate P2X7 and TNF-α in microglia/macrophages. P2x7 (also known as P2rx7), Tnf-α (also known as Tnf), Il-1β (also known as Il1b), Nos2, Icam-1 (also known as Icam1) and Ccl2 mRNA were not increased in these mice and the mice did not develop retinal leucostasis and capillary degeneration. Diabetic B6 mice treated intravitreally with a cell-permeable peptide that disrupts CD40-TRAF2,3 signalling did not exhibit either upregulation of P2X7 and inflammatory molecules in the retina or leucostasis.
Conclusions/interpretation: CD40-TRAF2,3 signalling activated the CD40-PLCγ1-ATP-P2X7-proinflammatory cytokine pathway. Src functioned as a link between CD40-TRAF2,3 and PLCγ1. Replacing WT CD40 with CD40 ΔT2,3 impaired activation of PLCγ1 in Müller cells, upregulation of P2X7 in microglia/macrophages, upregulation of a broad range of inflammatory molecules in the diabetic retina and the development of diabetic retinopathy. Administration of a peptide that disrupts CD40-TRAF2,3 signalling reduced retinal expression of inflammatory molecules and reduced leucostasis in diabetic mice, supporting the therapeutic potential of pharmacological inhibition of CD40-TRAF2,3 in diabetic retinopathy.
Keywords: CD40; Diabetic retinopathy; Endothelial cells; Inflammation; Microglia/macrophages; Müller cells.
© 2022. The Author(s).
Figures

References
-
- Portillo J-AC, Lopez Corcino Y, Miao Y, et al. CD40 in retinal Muller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy in mice. Diabetes. 2017;66:483–493. doi: 10.2337/db16-0051. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

