The effect of self-administered methamphetamine on GABAergic interneuron populations and functional connectivity of the nucleus accumbens and prefrontal cortex
- PMID: 35920922
- PMCID: PMC9385811
- DOI: 10.1007/s00213-022-06175-9
The effect of self-administered methamphetamine on GABAergic interneuron populations and functional connectivity of the nucleus accumbens and prefrontal cortex
Abstract
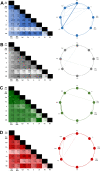
Introduction: Methamphetamine (METH, "ice") is a potent and addictive psychostimulant. Abuse of METH perturbs neurotransmitter systems and induces neurotoxicity; however, the neurobiological mechanisms which underlie addiction to METH are not fully understood, limiting the efficacy of available treatments. Here we investigate METH-induced changes to neuronal nitric oxide synthase (nNOS), parvalbumin and calretinin-expressing GABAergic interneuron populations within the nucleus accumbens (NAc), prefrontal cortex (PFC) and orbitofrontal cortex (OFC). We hypothesise that dysfunction or loss of these GABAergic interneuron populations may disrupt the excitatory/inhibitory balance within the brain.
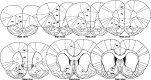
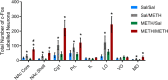
Methods: Male Long Evans rats (N = 32) were trained to lever press for intravenous METH or received yoked saline infusions. Following 14 days of behavioural extinction, animals were given a non-contingent injection of saline or METH (1 mg/kg, IP) to examine drug-primed reinstatement to METH-seeking behaviours. Ninety minutes post-IP injection, animals were culled and brain sections were analysed for Fos, nNOS, parvalbumin and calretinin immunoreactivity in eight distinct subregions of the NAc, PFC and OFC.
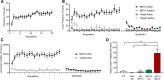
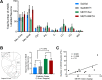
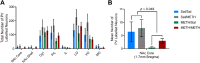
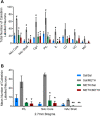
Results: METH exposure differentially affected GABAergic populations, with METH self-administration increasing nNOS immunoreactivity at distinct locations in the prelimbic cortex and decreasing parvalbumin immunoreactivity in the NAc. METH self-administration triggered reduced calretinin immunoreactivity, whilst acute METH administration produced a significant increase in calretinin immunoreactivity. As expected, non-contingent METH-priming treatment increased Fos immunoreactivity in subregions of the NAc and PFC.
Conclusion: Here we report that METH exposure in this model may alter the function of GABAergic interneurons in more subtle ways, such as alterations in neuronal firing or synaptic connectivity.
Keywords: Calretinin; Drugs of abuse; GABAergic interneurons; Methamphetamine; Methamphetamine-induced neuroadaptations; Neuronal nitric oxide synthase; Parvalbumin; Relapse to methamphetamine; Self-administration.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Oxytocin treatment in the prelimbic cortex reduces relapse to methamphetamine-seeking and is associated with reduced activity in the rostral nucleus accumbens core.Pharmacol Biochem Behav. 2019 Aug;183:64-71. doi: 10.1016/j.pbb.2019.06.002. Epub 2019 Jun 13. Pharmacol Biochem Behav. 2019. PMID: 31202809
-
Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats.Neuropsychopharmacology. 2014 Mar;39(4):811-22. doi: 10.1038/npp.2013.231. Epub 2013 Sep 2. Neuropsychopharmacology. 2014. PMID: 23995583 Free PMC article.
-
Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats.Addict Biol. 2016 Mar;21(2):316-25. doi: 10.1111/adb.12198. Epub 2014 Nov 16. Addict Biol. 2016. PMID: 25399704
-
Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review.
-
GABA system in the prefrontal cortex involved in psychostimulant addiction.Cereb Cortex. 2024 Aug 1;34(8):bhae319. doi: 10.1093/cercor/bhae319. Cereb Cortex. 2024. PMID: 39098820 Review.
Cited by
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
-
Decoding Secondary Motor Cortex Neuronal Activity During Cocaine Self-Administration: Insights From Longitudinal In Vivo Calcium Imaging.Biol Psychiatry Glob Open Sci. 2025 May 9;5(5):100531. doi: 10.1016/j.bpsgos.2025.100531. eCollection 2025 Sep. Biol Psychiatry Glob Open Sci. 2025. PMID: 40703665 Free PMC article.
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

