The multifaceted mechanisms of malignant glioblastoma progression and clinical implications
- PMID: 35920986
- PMCID: PMC9758111
- DOI: 10.1007/s10555-022-10051-5
The multifaceted mechanisms of malignant glioblastoma progression and clinical implications
Abstract
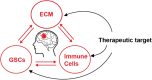
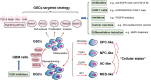
With the application of high throughput sequencing technologies at single-cell resolution, studies of the tumor microenvironment in glioblastoma, one of the most aggressive and invasive of all cancers, have revealed immense cellular and tissue heterogeneity. A unique extracellular scaffold system adapts to and supports progressive infiltration and migration of tumor cells, which is characterized by altered composition, effector delivery, and mechanical properties. The spatiotemporal interactions between malignant and immune cells generate an immunosuppressive microenvironment, contributing to the failure of effective anti-tumor immune attack. Among the heterogeneous tumor cell subpopulations of glioblastoma, glioma stem cells (GSCs), which exhibit tumorigenic properties and strong invasive capacity, are critical for tumor growth and are believed to contribute to therapeutic resistance and tumor recurrence. Here we discuss the role of extracellular matrix and immune cell populations, major components of the tumor ecosystem in glioblastoma, as well as signaling pathways that regulate GSC maintenance and invasion. We also highlight emerging advances in therapeutic targeting of these components.
Keywords: Extracellular matrix; Glioblastoma; Glioma stem cell; Immune cell; Invasion; Tumor microenvironment.
© 2022. The Author(s).
Conflict of interest statement
A.H.K. is a consultant for Monteris Medical.
Figures



References
-
- Stupp, R., Hegi, M. E., Mason, W. P., van den Bent, M. J., Taphoorn, M. J., Janzer, R. C., Group, N. C. I. o. C. C. T Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet Oncology. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

