Exploring Links Between Psychosis and Frontotemporal Dementia Using Multimodal Machine Learning: Dementia Praecox Revisited
- PMID: 35921104
- PMCID: PMC9350851
- DOI: 10.1001/jamapsychiatry.2022.2075
Exploring Links Between Psychosis and Frontotemporal Dementia Using Multimodal Machine Learning: Dementia Praecox Revisited
Abstract
Importance: The behavioral and cognitive symptoms of severe psychotic disorders overlap with those seen in dementia. However, shared brain alterations remain disputed, and their relevance for patients in at-risk disease stages has not been explored so far.
Objective: To use machine learning to compare the expression of structural magnetic resonance imaging (MRI) patterns of behavioral-variant frontotemporal dementia (bvFTD), Alzheimer disease (AD), and schizophrenia; estimate predictability in patients with bvFTD and schizophrenia based on sociodemographic, clinical, and biological data; and examine prognostic value, genetic underpinnings, and progression in patients with clinical high-risk (CHR) states for psychosis or recent-onset depression (ROD).
Design, setting, and participants: This study included 1870 individuals from 5 cohorts, including (1) patients with bvFTD (n = 108), established AD (n = 44), mild cognitive impairment or early-stage AD (n = 96), schizophrenia (n = 157), or major depression (n = 102) to derive and compare diagnostic patterns and (2) patients with CHR (n = 160) or ROD (n = 161) to test patterns' prognostic relevance and progression. Healthy individuals (n = 1042) were used for age-related and cohort-related data calibration. Data were collected from January 1996 to July 2019 and analyzed between April 2020 and April 2022.
Main outcomes and measures: Case assignments based on diagnostic patterns; sociodemographic, clinical, and biological data; 2-year functional outcomes and genetic separability of patients with CHR and ROD with high vs low pattern expression; and pattern progression from baseline to follow-up MRI scans in patients with nonrecovery vs preserved recovery.
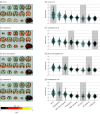
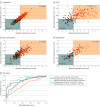
Results: Of 1870 included patients, 902 (48.2%) were female, and the mean (SD) age was 38.0 (19.3) years. The bvFTD pattern comprising prefrontal, insular, and limbic volume reductions was more expressed in patients with schizophrenia (65 of 157 [41.2%]) and major depression (22 of 102 [21.6%]) than the temporo-limbic AD patterns (28 of 157 [17.8%] and 3 of 102 [2.9%], respectively). bvFTD expression was predicted by high body mass index, psychomotor slowing, affective disinhibition, and paranoid ideation (R2 = 0.11). The schizophrenia pattern was expressed in 92 of 108 patients (85.5%) with bvFTD and was linked to the C9orf72 variant, oligoclonal banding in the cerebrospinal fluid, cognitive impairment, and younger age (R2 = 0.29). bvFTD and schizophrenia pattern expressions forecasted 2-year psychosocial impairments in patients with CHR and were predicted by polygenic risk scores for frontotemporal dementia, AD, and schizophrenia. Findings were not associated with AD or accelerated brain aging. Finally, 1-year bvFTD/schizophrenia pattern progression distinguished patients with nonrecovery from those with preserved recovery.
Conclusions and relevance: Neurobiological links may exist between bvFTD and psychosis focusing on prefrontal and salience system alterations. Further transdiagnostic investigations are needed to identify shared pathophysiological processes underlying the neuroanatomical interface between the 2 disease spectra.
Conflict of interest statement
Figures


References
-
- Bleuler E. Dementia Praecox Oder Gruppe Der Schizophrenien. Deuticke; 1911. - PubMed
-
- Kraepelin E. Dementia Praecox and Paraphrenia. Chicago Medical Book Co; 1919.
-
- Plum F. Prospects for research on schizophrenia. 3. neurophysiology. neuropathological findings. Neurosci Res Program Bull. 1972;10(4):384-388. - PubMed

