Interactions between Nitric Oxide and Hyaluronan Implicate the Migration of Breast Cancer Cells
- PMID: 35921128
- PMCID: PMC9472231
- DOI: 10.1021/acs.biomac.2c00545
Interactions between Nitric Oxide and Hyaluronan Implicate the Migration of Breast Cancer Cells
Abstract
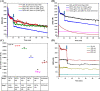
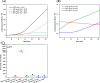
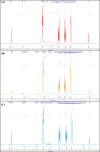
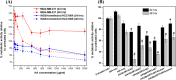
Nitric oxide (•NO) is one of the prominent free radicals, playing a pivotal role in breast cancer progression. Hyaluronic acid (HA) plays an essential role in neutralizing free radicals in tumor tissues. However, its interactions with nitric oxide have not been thoroughly investigated. Hence, this study attempts to understand the mechanism of these interactions and the different effects on the intracellular •NO levels and migration of breast cancer cells. The affinity of HA to scavenge •NO was investigated alongside the accompanying changes in specific physico-chemical properties and the further effects on the •NO-induced attachment and migration of the breast cancer cell lines, MDA-MB-231 and HCC1806. The reaction of the nitrogen dioxide radical, formed via •NO/O2 interactions, with HA initiated a series of oxidative reactions, which, in the presence of •NO, induce the fragmentation of the polymeric chains. Furthermore, these interactions were found to hinder the NO-induced migration of cancer cells. However, the NO-induced HA modification/fragmentation was inhibited in the presence of hemin, a NO-scavenging compound. Collectively, these results help toward understanding the involvement of HA in the •NO-induced cell migration and suggest the possible modification of HA, used as one of the main materials in different biomedical applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







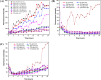
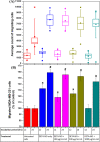
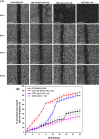
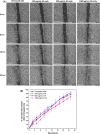
References
-
- Vigetti D.; Karousou E.; Viola M.; Passi A.; Analysis of Hyaluronan Synthase Activity. Methods in Molecular Biology; Humana Press: New York, NY, 2015; Vol. 1229, pp 201–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

