Targeted Whole Exome Sequencing in Children With Early-Onset Epilepsy: Parent Experiences
- PMID: 35921196
- PMCID: PMC9554160
- DOI: 10.1177/08830738221113901
Targeted Whole Exome Sequencing in Children With Early-Onset Epilepsy: Parent Experiences
Abstract
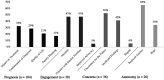
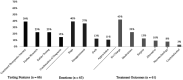
This study investigated the experiences of 25 caregivers of children with early-onset, treatment-resistant epilepsy who pursued whole exome sequencing to determine the impact of the test results on their child's treatment. Caregivers who consented to be recontacted were recruited from a previous study investigating the diagnostic yield of whole exome sequencing. A semistructured interview addressed questions based on one of 2 study phases. The first phase discussed the decision-making process for genetic testing (15 interviews), which revealed 4 major themes: (1) prognosis, (2) engagement, (3) concerns, and (4) autonomy. The second phase discussed the impact of genetic testing on treatment (10 interviews), which revealed 3 major themes: (1) testing features, (2) emotional impact, and (3) treatment outcomes. Overall, parents pursued genetic testing to obtain a clear prognosis, inform treatment decisions, engage with other families, and exercise autonomy. Caregivers felt that early testing is warranted to inform their child's diagnostic odyssey.
Keywords: and treatment; children; epilepsy; ethics; genetics; mutation; next-generation sequencing; outcome; seizures.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.