Impact of Epstein-Barr virus co-infection on natural acquired Plasmodium vivax antibody response
- PMID: 35921373
- PMCID: PMC9377613
- DOI: 10.1371/journal.pntd.0010305
Impact of Epstein-Barr virus co-infection on natural acquired Plasmodium vivax antibody response
Abstract
Background: The simultaneous infection of Plasmodium falciparum and Epstein-Barr virus (EBV) could promote the development of the aggressive endemic Burkitt's Lymphoma (eBL) in children living in P. falciparum holoendemic areas. While it is well-established that eBL is not related to other human malaria parasites, the impact of EBV infection on the generation of human malaria immunity remains largely unexplored. Considering that this highly prevalent herpesvirus establishes a lifelong persistent infection on B-cells with possible influence on malaria immunity, we hypothesized that EBV co-infection could have impact on the naturally acquired antibody responses to P. vivax, the most widespread human malaria parasite.
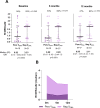
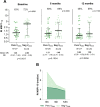
Methodology/principal findings: The study design involved three cross-sectional surveys at six-month intervals (baseline, 6 and 12 months) among long-term P. vivax exposed individuals living in the Amazon rainforest. The approach focused on a group of malaria-exposed individuals whose EBV-DNA (amplification of balf-5 gene) was persistently detected in the peripheral blood (PersVDNA, n = 27), and an age-matched malaria-exposed group whose EBV-DNA could never be detected during the follow-up (NegVDNA, n = 29). During the follow-up period, the serological detection of EBV antibodies to lytic/ latent viral antigens showed that IgG antibodies to viral capsid antigen (VCA-p18) were significantly different between groups (PersVDNA > NegVDNA). A panel of blood-stage P. vivax antigens covering a wide range of immunogenicity confirmed that in general PersVDNA group showed low levels of antibodies as compared with NegVDNA. Interestingly, more significant differences were observed to a novel DBPII immunogen, named DEKnull-2, which has been associated with long-term neutralizing antibody response. Differences between groups were less pronounced with blood-stage antigens (such as MSP1-19) whose levels can fluctuate according to malaria transmission.
Conclusions/significance: In a proof-of-concept study we provide evidence that a persistent detection of EBV-DNA in peripheral blood of adults in a P. vivax semi-immune population may impact the long-term immune response to major malaria vaccine candidates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, et al. Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77(6 Suppl):88–98. Epub 2008/01/31. ; PubMed Central PMCID: PMC2637949. - PMC - PubMed
-
- Afolabi MO, Ale BM, Dabira ED, Agbla SC, Bustinduy AL, Ndiaye JLA, et al. Malaria and helminth co-infections in children living in endemic countries: A systematic review with meta-analysis. PLoS Negl Trop Dis. 2021;15(2):e0009138. Epub 2021/02/19. doi: 10.1371/journal.pntd.0009138 ; PubMed Central PMCID: PMC7924789. - DOI - PMC - PubMed
-
- Nyirenda TS, Mandala WL, Gordon MA, Mastroeni P. Immunological bases of increased susceptibility to invasive nontyphoidal Salmonella infection in children with malaria and anaemia. Microbes Infect. 2018;20(9–10):589–98. Epub 2017/12/19. doi: 10.1016/j.micinf.2017.11.014 ; PubMed Central PMCID: PMC6250906. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

