Regulation of membrane homeostasis by TMC1 mechanoelectrical transduction channels is essential for hearing
- PMID: 35921424
- PMCID: PMC9348795
- DOI: 10.1126/sciadv.abm5550
Regulation of membrane homeostasis by TMC1 mechanoelectrical transduction channels is essential for hearing
Abstract
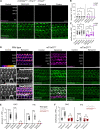
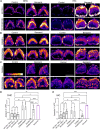
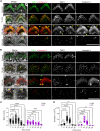
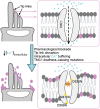
The mechanoelectrical transduction (MET) channel in auditory hair cells converts sound into electrical signals, enabling hearing. Transmembrane-like channel 1 and 2 (TMC1 and TMC2) are implicated in forming the pore of the MET channel. Here, we demonstrate that inhibition of MET channels, breakage of the tip links required for MET, or buffering of intracellular Ca... induces pronounced phosphatidylserine externalization, membrane blebbing, and ectosome release at the hair cell sensory organelle, culminating in the loss of TMC1. Membrane homeostasis triggered by MET channel inhibition requires Tmc1 but not Tmc2, and three deafness-causing mutations in Tmc1 cause constitutive phosphatidylserine externalization that correlates with deafness phenotype. Our results suggest that, in addition to forming the pore of the MET channel, TMC1 is a critical regulator of membrane homeostasis in hair cells, and that Tmc1-related hearing loss may involve alterations in membrane homeostasis.
Figures




References
-
- Assad J. A., Shepherd G. M., Corey D. P., Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994 (1991). - PubMed
-
- Pan B., Akyuz N., Liu X. P., Asai Y., Nist-Lund C., Kurima K., Derfler B. H., Gyorgy B., Limapichat W., Walujkar S., Wimalasena L. N., Sotomayor M., Corey D. P., Holt J. R., TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99, 736–753.e6 (2018). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

