Targeting acetyl-CoA metabolism attenuates the formation of fear memories through reduced activity-dependent histone acetylation
- PMID: 35921439
- PMCID: PMC9371679
- DOI: 10.1073/pnas.2114758119
Targeting acetyl-CoA metabolism attenuates the formation of fear memories through reduced activity-dependent histone acetylation
Abstract
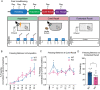
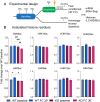
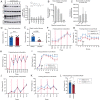
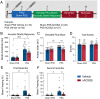
Histone acetylation is a key component in the consolidation of long-term fear memories. Histone acetylation is fueled by acetyl-coenzyme A (acetyl-CoA), and recently, nuclear-localized metabolic enzymes that produce this metabolite have emerged as direct and local regulators of chromatin. In particular, acetyl-CoA synthetase 2 (ACSS2) mediates histone acetylation in the mouse hippocampus. However, whether ACSS2 regulates long-term fear memory remains to be determined. Here, we show that Acss2 knockout is well tolerated in mice, yet the Acss2-null mouse exhibits reduced acquisition of long-term fear memory. Loss of Acss2 leads to reductions in both histone acetylation and expression of critical learning and memory-related genes in the dorsal hippocampus, specifically following fear conditioning. Furthermore, systemic administration of blood-brain barrier-permeable Acss2 inhibitors during the consolidation window reduces fear-memory formation in mice and rats and reduces anxiety in a predator-scent stress paradigm. Our findings suggest that nuclear acetyl-CoA metabolism via ACSS2 plays a critical, previously unappreciated, role in the formation of fear memories.
Keywords: epigenetics; fear conditioning; histone acetylation; learning and memory; mass spectrometry.
Conflict of interest statement
Competing interest statement: S.L.B. and P.M. are cofounders of EpiVario, Inc. T.K. is the chief executive officer of EpiVario. EpiVario provided experimental compounds (ACSS2i) through a sponsored research agreement with the University of Pennsylvania.
Figures


References
-
- Levenson J. M., et al. , Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279, 40545–40559 (2004). - PubMed
-
- Kandel E. R., Dudai Y., Mayford M. R., The molecular and systems biology of memory. Cell 157, 163–186 (2014). - PubMed
-
- Gräff J., Tsai L. H., The potential of HDAC inhibitors as cognitive enhancers. Annu. Rev. Pharmacol. Toxicol. 53, 311–330 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

