Clonal germinal center B cells function as a niche for T-cell lymphoma
- PMID: 35921527
- PMCID: PMC10653021
- DOI: 10.1182/blood.2022015451
Clonal germinal center B cells function as a niche for T-cell lymphoma
Abstract
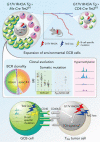
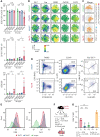
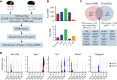
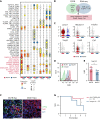
Angioimmunoblastic T-cell lymphoma (AITL) is proposed to be initiated by age-related clonal hematopoiesis (ACH) with TET2 mutations, whereas the G17V RHOA mutation in immature cells with TET2 mutations promotes the development of T follicular helper (TFH)-like tumor cells. Here, we investigated the mechanism by which TET2-mutant immune cells enable AITL development using mouse models and human samples. Among the 2 mouse models, mice lacking Tet2 in all the blood cells (Mx-Cre × Tet2flox/flox × G17V RHOA transgenic mice) spontaneously developed AITL for approximately up to a year, while mice lacking Tet2 only in the T cells (Cd4-Cre × Tet2flox/flox × G17V RHOA transgenic mice) did not. Therefore, Tet2-deficient immune cells function as a niche for AITL development. Single-cell RNA-sequencing (scRNA-seq) of >50 000 cells from mouse and human AITL samples revealed significant expansion of aberrant B cells, exhibiting properties of activating light zone (LZ)-like and proliferative dark zone (DZ)-like germinal center B (GCB) cells. The GCB cells in AITL clonally evolved with recurrent mutations in genes related to core histones. In silico network analysis using scRNA-seq data identified Cd40-Cd40lg as a possible mediator of GCB and tumor cell cluster interactions. Treatment of AITL model mice with anti-Cd40lg inhibitory antibody prolonged survival. The genes expressed in aberrantly expanded GCB cells in murine tumors were also broadly expressed in the B-lineage cells of TET2-mutant human AITL. Therefore, ACH-derived GCB cells could undergo independent clonal evolution and support the tumorigenesis in AITL via the CD40-CD40LG axis.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
A Tet-a-Tet in T follicular helper cell lymphoma.Blood. 2022 Nov 3;140(18):1919-1921. doi: 10.1182/blood.2022017807. Blood. 2022. PMID: 36326793 No abstract available.
References
-
- Swerdlow SH, Campo E, Harris NL, et al. In: World Health Organization Classification of Tumours. 4th ed. Swerdlow SH, Campo E, Harris NL, et al., editors. Vol. 2. IARC; Lyon, France: 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. (Revised).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

