Genome-wide CRISPR screens identify CD48 defining susceptibility to NK cytotoxicity in peripheral T-cell lymphomas
- PMID: 35921533
- PMCID: PMC9837448
- DOI: 10.1182/blood.2022015646
Genome-wide CRISPR screens identify CD48 defining susceptibility to NK cytotoxicity in peripheral T-cell lymphomas
Abstract
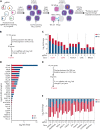
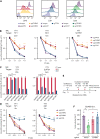
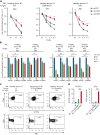
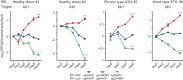
Adult T-cell leukemia/lymphoma (ATLL) is one of the aggressive peripheral T-cell neoplasms with a poor prognosis. Accumulating evidence demonstrates that escape from adaptive immunity is a hallmark of ATLL pathogenesis. However, the mechanisms by which ATLL cells evade natural killer (NK)-cell-mediated immunity have been poorly understood. Here we show that CD48 expression in ATLL cells determines the sensitivity for NK-cell-mediated cytotoxicity against ATLL cells. We performed unbiased genome-wide clustered regularly interspaced short palindromic repeat (CRISPR) screening using 2 ATLL-derived cell lines and discovered CD48 as one of the best-enriched genes whose knockout conferred resistance to YT1-NK cell line-mediated cytotoxicity. The ability of CD48-knockout ATLL cells to evade NK-cell effector function was confirmed using human primary NK cells with reduced interferon-γ (IFNγ) induction and degranulation. We found that primary ATLL cells had reduced CD48 expression along with disease progression. Furthermore, other subgroups among aggressive peripheral T-cell lymphomas (PTCLs) also expressed lower concentrations of CD48 than normal T cells, suggesting that CD48 is a key molecule in malignant T-cell evasion of NK-cell surveillance. Thus, this study demonstrates that CD48 expression is likely critical for malignant T-cell lymphoma cell regulation of NK-cell-mediated immunity and provides a rationale for future evaluation of CD48 as a molecular biomarker in NK-cell-associated immunotherapies.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



References
-
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–280. - PubMed
-
- Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47(11):1304–1315. - PubMed
-
- Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534(7607):402–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

