Efficacy of neoadjuvant treatment with or without pertuzumab in patients with stage II and III HER2-positive breast cancer: a nationwide cohort analysis of pathologic response and 5-year survival
- PMID: 35921798
- PMCID: PMC9356182
- DOI: 10.1016/j.breast.2022.07.005
Efficacy of neoadjuvant treatment with or without pertuzumab in patients with stage II and III HER2-positive breast cancer: a nationwide cohort analysis of pathologic response and 5-year survival
Abstract
Background: Pathologic complete response (pCR) rates in early stage HER2-positive breast cancer improved after pertuzumab was added to neoadjuvant treatment. However, survival benefit is less-well established and seems mostly limited to node-positive patients. We used national cancer registry data to compare outcomes of patients treated with and without pertuzumab.
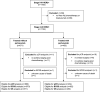
Methods: We identified stage II-III HER2-positive breast cancer patients treated with neoadjuvant trastuzumab-based chemotherapy between November 2013 until January 2016 from the Netherlands Cancer Registry. During that period pertuzumab was only available in the 37 hospitals that participated in the TRAIN-2 study. Missing grade and pCR-status were obtained from the Dutch Pathology Registry (PALGA) and cause of death from Statistics Netherlands. We used multiple imputation to impute missing data, multivariable logistic regression to evaluate the association between pertuzumab and pCR (ypT0/is, ypN0) and multivariable Cox regression models for overall survival and breast cancer specific survival (BCSS).
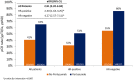
Results: We identified 1124 patients of whom 453 received pertuzumab. Baseline characteristics were comparable, although tumor grade was missing more often in patients treated without pertuzumab (12% vs. 2%). Pertuzumab improved pCR rates (41% vs 65%, adjusted odds ratio [aOR] 2.91; 95% CI:2.20-3.94). After a median follow-up of 6.0 years, 5-year BCSS rates were 95% and 98% respectively (adjusted hazard ratio [aHR]: 0.58; 95% CI:0.36-0.95). Younger patients derived more benefit from pertuzumab, but no other significant interactions were found.
Conclusion: These results support earlier data of a small survival benefit with the addition of pertuzumab to trastuzumab-based neoadjuvant chemotherapy which is most meaningful in younger patients.
Keywords: ErbB2; HER2-Positive; Neoadjuvant chemotherapy; Non-metastatic breast cancer; Pertuzumab; Survival.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest GSS reports institutional research support from Agendia, AstraZeneca, Merck, Novartis, Roche and Seagen and consultancy fees paid to the institution from Biovica and Seagen. All other authors declare that they have no conflicts of interest.
Figures


References
-
- Gianni L., Pienkowski T., Im Y.H., Roman L., Tseng L.M., Liu M.C., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. - PubMed
-
- Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. - PubMed
-
- Swain S.M., Ewer M.S., Viale G., Delaloge S., Ferrero J.M., Verrill M., et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–653. - PMC - PubMed
-
- van Ramshorst M.S., van der Voort A., van Werkhoven E.D., Mandjes I.A., Kemper I., Dezentje V.O., et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630–1640. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

