Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue
- PMID: 35921984
- PMCID: PMC9396640
- DOI: 10.1016/j.molmet.2022.101550
Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue
Abstract
Objectives: Tirzepatide, a dual GIP and GLP-1 receptor agonist, delivered superior glycemic control and weight loss compared to selective GLP-1 receptor (GLP-1R) agonism in patients with type 2 diabetes (T2D). These results have fueled mechanistic studies focused on understanding how tirzepatide achieves its therapeutic efficacy. Recently, we found that treatment with tirzepatide improves insulin sensitivity in humans with T2D and obese mice in concert with a reduction in circulating levels of branched-chain amino (BCAAs) and keto (BCKAs) acids, metabolites associated with development of systemic insulin resistance (IR) and T2D. Importantly, these systemic effects were found to be coupled to increased expression of BCAA catabolic genes in thermogenic brown adipose tissue (BAT) in mice. These findings led us to hypothesize that tirzepatide may lower circulating BCAAs/BCKAs by promoting their catabolism in BAT.
Methods: To address this question, we utilized a murine model of diet-induced obesity and employed stable-isotope tracer studies in combination with metabolomic analyses in BAT and other tissues.
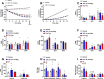
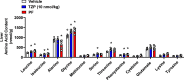
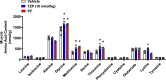
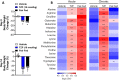
Results: Treatment with tirzepatide stimulated catabolism of BCAAs/BCKAs in BAT, as demonstrated by increased labeling of BCKA-derived metabolites, and increases in levels of byproducts of BCAA breakdown, including glutamate, alanine, and 3-hydroxyisobutyric acid (3-HIB). Further, chronic administration of tirzepatide increased levels of multiple amino acids in BAT that have previously been shown to be elevated in response to cold exposure. Finally, chronic treatment with tirzepatide led to a substantial increase in several TCA cycle intermediates (α-ketoglutarate, fumarate, and malate) in BAT.
Conclusions: These findings suggest that tirzepatide induces a thermogenic-like amino acid profile in BAT, an effect that may account for reduced systemic levels of BCAAs in obese IR mice.
Keywords: BAT; BCAAs; BCKAs; GIPR; GLP-1R; Tirzepatide.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 2019;15(5):288–298. - PubMed
-
- Zimmet P., Alberti K.G., Magliano D.J., Bennett P.H. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nature Reviews Endocrinology. 2016;12(10):616–622. - PubMed
-
- Tschöp M.H., Finan B., Clemmensen C., Gelfanov V., Perez-Tilve D., Müller T.D., et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metabolism. 2016;24(1):51–62. - PubMed
-
- Samms R.J., Coghlan M.P., Sloop K.W. How may GIP enhance the therapeutic efficacy of GLP-1? Trends in Endocrinology and Metabolism. 2020;31(6):410–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

