Development and validation of an early warning score to identify COVID-19 in the emergency department based on routine laboratory tests: a multicentre case-control study
- PMID: 35922102
- PMCID: PMC9352566
- DOI: 10.1136/bmjopen-2021-059111
Development and validation of an early warning score to identify COVID-19 in the emergency department based on routine laboratory tests: a multicentre case-control study
Abstract
Objectives: Identifying patients with a possible SARS-CoV-2 infection in the emergency department (ED) is challenging. Symptoms differ, incidence rates vary and test capacity may be limited. As PCR-testing all ED patients is neither feasible nor effective in most centres, a rapid, objective, low-cost early warning score to triage ED patients for a possible infection is developed.
Design: Case-control study.
Setting: Secondary and tertiary hospitals in the Netherlands.
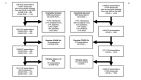
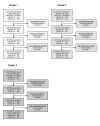
Participants: The study included patients presenting to the ED with venous blood sampling from July 2019 to July 2020 (n=10 417, 279 SARS-CoV-2-positive). The temporal validation cohort covered the period from July 2020 to October 2021 (n=14 080, 1093 SARS-CoV-2-positive). The external validation cohort consisted of patients presenting to the ED of three hospitals in the Netherlands (n=12 061, 652 SARS-CoV-2-positive).
Primary outcome measures: The primary outcome was one or more positive SARS-CoV-2 PCR test results within 1 day prior to or 1 week after ED presentation.
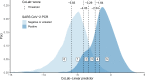
Results: The resulting 'CoLab-score' consists of 10 routine laboratory measurements and age. The score showed good discriminative ability (AUC: 0.930, 95% CI 0.909 to 0.945). The lowest CoLab-score had high sensitivity for COVID-19 (0.984, 95% CI 0.970 to 0.991; specificity: 0.411, 95% CI 0.285 to 0.520). Conversely, the highest score had high specificity (0.978, 95% CI 0.973 to 0.983; sensitivity: 0.608, 95% CI 0.522 to 0.685). The results were confirmed in temporal and external validation.
Conclusions: The CoLab-score is based on routine laboratory measurements and is available within 1 hour after presentation. Depending on the prevalence, COVID-19 may be safely ruled out in over one-third of ED presentations. Highly suspect cases can be identified regardless of presenting symptoms. The CoLab-score is continuous, in contrast to the binary outcome of lateral flow testing, and can guide PCR testing and triage ED patients.
Keywords: COVID-19; Clinical chemistry; Health informatics; accident & emergency medicine; statistics & research methods.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Disease C. (COVID-19) situation reports. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio... [Accessed 4 Feb 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
