DNA Methylation Analysis to predict Regression of high-grade anal Intraepithelial Neoplasia in HIV+ men (MARINE): a cohort study protocol
- PMID: 35922105
- PMCID: PMC9352988
- DOI: 10.1136/bmjopen-2021-060301
DNA Methylation Analysis to predict Regression of high-grade anal Intraepithelial Neoplasia in HIV+ men (MARINE): a cohort study protocol
Abstract
Introduction: Anal cancer precursors, or high-grade anal intraepithelial neoplasia (HGAIN), are highly prevalent in HIV-seropositive (HIV+) men who have sex with men (MSM). Around 30% of lesions regress within 1 year, but current histopathological assessment is unable to distinguish between HGAIN likely to regress and HGAIN likely to persist or progress to cancer. We aim to assess if host cell DNA methylation markers can predict regression of HGAIN, thus determining the need for immediate treatment or active surveillance. This could reduce overtreatment and the associated anal and psycho-sexual morbidity.
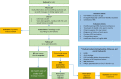
Methods and analysis: This is an active surveillance cohort study in three centres located in Amsterdam, the Netherlands, in 200 HIV+ MSM diagnosed with HGAIN. Participants will not be treated, but closely monitored during 24 months of follow-up with 6 monthly visits including cytology, and high-resolution anoscopy with biopsies. The primary study endpoint is histopathological regression of each baseline HGAIN lesion at the end of the study. Regression is defined as ≤low grade anal intraepithelial neoplasia in the exit biopsy at 24 months. Regression proportions in lesions with low versus high methylation levels (ASCL1, ZNF582), other biomarkers (HPV genotype, HPV-E4, p16INK4A, Ki-67) and immunological markers at baseline will be compared. Main secondary endpoints are the histological and clinical outcome (ie, the number of octants affected by HGAIN) of each baseline HGAIN lesion and overall HGAIN disease (i.e., all lesions combined) after each visit. The health-related quality of life of the study group will be compared with that of a control group of 50 HIV+ MSM receiving regular HGAIN treatment.
Ethics and dissemination: Ethics approval was obtained from the Institutional Review Board of the Academic Medical Center (Amsterdam, The Netherlands; reference no. 2021_099). Participants are required to provide written informed consent. Findings will be disseminated through publication in peer-reviewed scientific journals and presentations at international scientific conferences; dissemination to policy makers and the target patient group will be achieved through our (inter-)national network, professional associations and collaboration with a patient representative organisation.
Trial registration number: NL9664.
Keywords: HIV & AIDS; dermatological tumours; dermatopathology; gastrointestinal tumours; infectious diseases & infestations; molecular diagnostics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RDMS is minority stockholder of Self-screen, a spin-off company of VUmc, which owns patents on methylation markers and HPV detection. HJCdV received financial compensation or goods for research from Medigene, Gilead and MSD; financial compensation for presentations from Abbott and Janssen and financial compensation for advice to Medigene and Novartis. MFSvdL served on advisory boards of Merck. All other authors reported no potential conflicts.
Figures
Similar articles
-
Cancer Risk Stratification of Anal Intraepithelial Neoplasia in Human Immunodeficiency Virus-Positive Men by Validated Methylation Markers Associated With Progression to Cancer.Clin Infect Dis. 2021 Jun 15;72(12):2154-2163. doi: 10.1093/cid/ciaa397. Clin Infect Dis. 2021. PMID: 32266940 Free PMC article.
-
HPV vaccination to prevent recurrence of anal intraepithelial neoplasia in HIV+ MSM.AIDS. 2021 Sep 1;35(11):1753-1764. doi: 10.1097/QAD.0000000000002928. AIDS. 2021. PMID: 33966029 Free PMC article. Clinical Trial.
-
Laser capture microdissection as a tool to evaluate human papillomavirus genotyping and methylation as biomarkers of persistence and progression of anal lesions.BMJ Open. 2015 Aug 26;5(8):e008439. doi: 10.1136/bmjopen-2015-008439. BMJ Open. 2015. PMID: 26310402 Free PMC article.
-
HPV and anal cancer in HIV-infected individuals: a review.Curr HIV/AIDS Rep. 2014 Sep;11(3):250-62. doi: 10.1007/s11904-014-0224-x. Curr HIV/AIDS Rep. 2014. PMID: 24990810 Review.
-
Human papillomavirus and anal neoplasia.Curr HIV/AIDS Rep. 2008 May;5(2):78-85. doi: 10.1007/s11904-008-0013-5. Curr HIV/AIDS Rep. 2008. PMID: 18510893 Review.
Cited by
-
Anal Cancer in High-Risk Women: The Lost Tribe.Cancers (Basel). 2022 Dec 22;15(1):60. doi: 10.3390/cancers15010060. Cancers (Basel). 2022. PMID: 36612055 Free PMC article. Review.
-
Anal Cancer and Anal Cancer Screening.Clin Obstet Gynecol. 2023 Sep 1;66(3):516-533. doi: 10.1097/GRF.0000000000000789. Epub 2023 Jul 13. Clin Obstet Gynecol. 2023. PMID: 37439541 Free PMC article.
-
Analytical validation and diagnostic performance of the ASCL1/ZNF582 methylation test for detection of high-grade anal intraepithelial neoplasia and anal cancer.Tumour Virus Res. 2024 Jun;17:200275. doi: 10.1016/j.tvr.2023.200275. Epub 2023 Dec 30. Tumour Virus Res. 2024. PMID: 38160718 Free PMC article.
-
Safety, feasibility, and short-term-outcome of anal endoscopic submucosal dissection for anal intraepithelial neoplasia: an option for focal lesions?Tech Coloproctol. 2023 Dec 15;28(1):18. doi: 10.1007/s10151-023-02896-x. Tech Coloproctol. 2023. PMID: 38102514 Free PMC article.
References
-
- Darragh TM, Colgan TJ, Cox JT, et al. . The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American pathologists and the American Society for colposcopy and cervical pathology. J Low Genit Tract Dis 2012;16:205–42. 10.1097/LGT.0b013e31825c31dd - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
