Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial
- PMID: 35922124
- PMCID: PMC9606533
- DOI: 10.1136/ard-2022-222405
Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial
Erratum in
-
Correction: Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label randomised controlled ORAL Surveillance trial.Ann Rheum Dis. 2023 Oct;82(10):e219. doi: 10.1136/ard-2022-222405corr1. Ann Rheum Dis. 2023. PMID: 37726134 Free PMC article. No abstract available.
Abstract
Objectives: To characterise infections in patients with rheumatoid arthritis (RA) in ORAL Surveillance.
Methods: In this open-label, randomised controlled trial, patients with RA aged≥50 years with ≥1 additional cardiovascular risk factor received tofacitinib 5 or 10 mg two times per day or a tumour necrosis factor inhibitor (TNFi). Incidence rates (IRs; patients with first events/100 patient-years) and hazard ratios (HRs) were calculated for infections, overall and by age (50-<65 years; ≥65 years). Probabilities of infections were obtained (Kaplan-Meier estimates). Cox modelling identified infection risk factors.
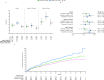
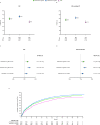
Results: IRs/HRs for all infections, serious infection events (SIEs) and non-serious infections (NSIs) were higher with tofacitinib (10>5 mg two times per day) versus TNFi. For SIEs, HR (95% CI) for tofacitinib 5 and 10 mg two times per day versus TNFi, respectively, were 1.17 (0.92 to 1.50) and 1.48 (1.17 to 1.87). Increased IRs/HRs for all infections and SIEs with tofacitinib 10 mg two times per day versus TNFi were more pronounced in patients aged≥65 vs 50-<65 years. SIE probability increased from month 18 and before month 6 with tofacitinib 5 and 10 mg two times per day versus TNFi, respectively. NSI probability increased before month 6 with both tofacitinib doses versus TNFi. Across treatments, the most predictive risk factors for SIEs were increasing age, baseline opioid use, history of chronic lung disease and time-dependent oral corticosteroid use, and, for NSIs, female sex, history of chronic lung disease/infections, past smoking and time-dependent Disease Activity Score in 28 joints, C-reactive protein.
Conclusions: Infections were higher with tofacitinib versus TNFi. Findings may inform future treatment decisions.
Trial registration number: NCT02092467.
Keywords: antirheumatic agents; arthritis, rheumatoid; therapeutics; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: A-RB has acted as a consultant for AbbVie, Akros, Alfasigma, Amgen, Biogen, Eli Lilly, MSD, Mylan, Novartis, Pfizer, Roche and UCB, has received speaker fees or honoraria from AbbVie, Alfasigma, Amgen, Angelini, AstraZeneca, Berlin-Chemie, Bristol-Myers Squibb, MSD, Novartis, Pfizer, Roche, Sandoz, Teva, UCB and Zentiva, and has been a principal investigator in studies sponsored by Akros, AstraZeneca, Bristol-Myers Squibb, GSK, MSD, Novartis, Pfizer, Roche and UCB. GC has received grants and/or research support from AbbVie, Amgen, Eli Lilly, Gema Pharma, Genzyme, Novartis, Pfizer and Sanofi-Genzyme, and has acted as a consultant for AbbVie, Amgen, Eli Lilly, Gema Pharma, Genzyme, Novartis, Pfizer and Sanofi-Genzyme. VP-R is an employee of Instituto Nacional de Ciencias Médicas y Nutrición and is a principal investigator in studies sponsored by Bristol-Myers Squibb and Pfizer.DLB is a member of the advisory board for: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; DLB/Brigham and Women's Hospital do not receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. He served as a member of the Steering Committee for ORAL Surveillance, with funding from Pfizer paid to Brigham and Women’s Hospital. CAC, DG and ABS are employees and stockholders of Pfizer. A-SC is an employee of Pfizer. GS is an employee of Syneos Health, who were paid contractors to Pfizer in the development of this manuscript. JEP has received grants and/or research support from Bristol-Myers Squibb, Roche, Seattle Genetics and UCB, and has acted as a consultant for AbbVie, Actelion, Amgen, Bayer, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi and UCB. HS-K has acted as a consultant for, and been an advisor or review panel member for, AbbVie, Eli Lilly, Galapagos, MSD, Pfizer and UCB.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

