Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study
- PMID: 35922128
- PMCID: PMC10313996
- DOI: 10.1136/thorax-2021-217032
Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study
Abstract
Rationale: Progressive lung function (LF) decline in patients with asthma contributes to worse outcomes. Asthma exacerbations are thought to contribute to this decline; however, evidence is limited with mixed results.
Methods: This historical cohort study of a broad asthma patient population in the Optimum Patient Care Research Database, examined asthma patients with 3+eligible post-18th birthday peak expiratory flow rate (PEF) records (primary analysis) or records of forced expiratory flow in 1 s (FEV1) (sensitivity analysis). Adjusted linear growth models tested the association between mean annual exacerbation rate (AER) and LF trajectory.
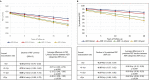
Results: We studied 1 09 182 patients with follow-up ranging from 5 to 50 years, of which 75 280 had data for all variables included in the adjusted analyses. For each additional exacerbation, an estimated additional -1.34 L/min PEF per year (95% CI -1.23 to -1.50) were lost. Patients with AERs >2/year and aged 18-24 years at baseline lost an additional -5.95 L/min PEF/year (95% CI -8.63 to -3.28) compared with those with AER 0. These differences in the rate of LF decline between AER groups became progressively smaller as age at baseline increased. The results using FEV1 were consistent with the above.
Conclusion: To our knowledge, this study is the largest nationwide cohort of its kind and demonstrates that asthma exacerbations are associated with faster LF decline. This was more prominent in younger patients but was evident in older patients when it was related to lower starting LF, suggesting a persistent deteriorating phenotype that develops in adulthood over time. Earlier intervention with appropriate management in younger patients with asthma could be of value to prevent excessive LF decline.
Keywords: asthma.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DS, VC and NE are employees of Optimum Patient Care, and SS, LB, IC and NH were employees of Optimum Patient Care. Optimum Patient Care is a co-funder of the International Severe Asthma Registry. LGH declares he has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Circassia, Hoffmann la Roche, GlaxoSmithKline, Novartis, and Teva; he has taken part in asthma clinical trials sponsored by Boehringer Ingelheim, Hoffmann la Roche, and GlaxoSmithKline for which his institution received remuneration; he is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche, and Janssen. TNT and BE are employees of AstraZeneca, and EGG was an employee of AstraZeneca. AstraZeneca is a co-funder of the International Severe Asthma Registry. AM-G has attended advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi and Teva, and has received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Roche, Teva and Vectura. He has participated in research with AstraZeneca for which his institution has been remunerated and has attended international conferences with Teva. He has had consultancy agreements with AstraZeneca, Sanofi, and Vectura. MP declares personal fees and non-financial support from AstraZeneca and GlaxoSmithKline. NL consulted for AstraZeneca and GSK; served on protocol committee with AstraZeneca; and served on advisory board with AstraZeneca, GSK, Sanofi, Novartis, Genentech and Teva. RJ reports grants, personal fees, and non-financial support from AstraZeneca and OPRI, personal fees and non-financial support from Boehringer Ingelheim, grants, personal fees, and non-financial support from GSK, grants and non-financial support from Novartis, non-financial support from Nutricia, and personal fees from Pfizer outside the submitted work. DBP has advisory board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
