Cellular recovery after prolonged warm ischaemia of the whole body
- PMID: 35922506
- PMCID: PMC9518831
- DOI: 10.1038/s41586-022-05016-1
Cellular recovery after prolonged warm ischaemia of the whole body
Abstract
After cessation of blood flow or similar ischaemic exposures, deleterious molecular cascades commence in mammalian cells, eventually leading to their death1,2. Yet with targeted interventions, these processes can be mitigated or reversed, even minutes or hours post mortem, as also reported in the isolated porcine brain using BrainEx technology3. To date, translating single-organ interventions to intact, whole-body applications remains hampered by circulatory and multisystem physiological challenges. Here we describe OrganEx, an adaptation of the BrainEx extracorporeal pulsatile-perfusion system and cytoprotective perfusate for porcine whole-body settings. After 1 h of warm ischaemia, OrganEx application preserved tissue integrity, decreased cell death and restored selected molecular and cellular processes across multiple vital organs. Commensurately, single-nucleus transcriptomic analysis revealed organ- and cell-type-specific gene expression patterns that are reflective of specific molecular and cellular repair processes. Our analysis comprises a comprehensive resource of cell-type-specific changes during defined ischaemic intervals and perfusion interventions spanning multiple organs, and it reveals an underappreciated potential for cellular recovery after prolonged whole-body warm ischaemia in a large mammal.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures
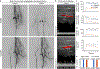













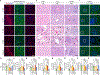
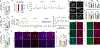

Comment in
-
Improved organ recovery after oxygen deprivation.Nature. 2022 Aug;608(7922):273-274. doi: 10.1038/d41586-022-01995-3. Nature. 2022. PMID: 35922486 No abstract available.
-
Cellular recovery after prolonged warm ischaemia.Nat Rev Nephrol. 2022 Oct;18(10):605. doi: 10.1038/s41581-022-00629-1. Nat Rev Nephrol. 2022. PMID: 36042350 No abstract available.
-
OrganEx: A Promising Solution for Rescuing Organs After Prolonged Ischemia.Gastroenterology. 2023 Apr;164(4):696-697. doi: 10.1053/j.gastro.2022.11.016. Epub 2022 Nov 15. Gastroenterology. 2023. PMID: 36400180 No abstract available.
References
-
- Daniele SG et al. Brain vulnerability and viability after ischaemia. Nat. Rev. Neurosci. 22, 553–572 (2021). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

