Brown-fat-mediated tumour suppression by cold-altered global metabolism
- PMID: 35922508
- PMCID: PMC9365697
- DOI: 10.1038/s41586-022-05030-3
Brown-fat-mediated tumour suppression by cold-altered global metabolism
Abstract
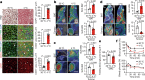
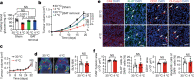
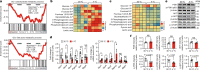
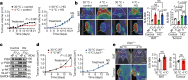
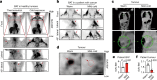
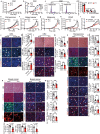
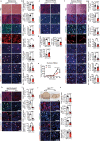
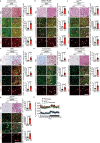
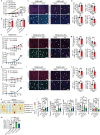
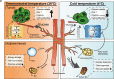
Glucose uptake is essential for cancer glycolysis and is involved in non-shivering thermogenesis of adipose tissues1-6. Most cancers use glycolysis to harness energy for their infinite growth, invasion and metastasis2,7,8. Activation of thermogenic metabolism in brown adipose tissue (BAT) by cold and drugs instigates blood glucose uptake in adipocytes4,5,9. However, the functional effects of the global metabolic changes associated with BAT activation on tumour growth are unclear. Here we show that exposure of tumour-bearing mice to cold conditions markedly inhibits the growth of various types of solid tumours, including clinically untreatable cancers such as pancreatic cancers. Mechanistically, cold-induced BAT activation substantially decreases blood glucose and impedes the glycolysis-based metabolism in cancer cells. The removal of BAT and feeding on a high-glucose diet under cold exposure restore tumour growth, and genetic deletion of Ucp1-the key mediator for BAT-thermogenesis-ablates the cold-triggered anticancer effect. In a pilot human study, mild cold exposure activates a substantial amount of BAT in both healthy humans and a patient with cancer with mitigated glucose uptake in the tumour tissue. These findings provide a previously undescribed concept and paradigm for cancer therapy that uses a simple and effective approach. We anticipate that cold exposure and activation of BAT through any other approach, such as drugs and devices either alone or in combination with other anticancer therapeutics, will provide a general approach for the effective treatment of various cancers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Cold-induced BAT stunts tumour growth.Nat Rev Endocrinol. 2022 Oct;18(10):587. doi: 10.1038/s41574-022-00738-8. Nat Rev Endocrinol. 2022. PMID: 36008623 No abstract available.
-
Live cold to grow old? Thermogenesis to fight cancer.Cell Res. 2022 Dec;32(12):1042-1043. doi: 10.1038/s41422-022-00723-w. Cell Res. 2022. PMID: 36085369 Free PMC article. No abstract available.
-
Starving cancer into submission by activating BAT.Cell Metab. 2022 Oct 4;34(10):1428-1430. doi: 10.1016/j.cmet.2022.09.009. Cell Metab. 2022. PMID: 36198290
-
Cold exposure as anti-cancer therapy.Cancer Cell. 2022 Oct 10;40(10):1092-1094. doi: 10.1016/j.ccell.2022.09.008. Cancer Cell. 2022. PMID: 36220071
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

