Efficacy and safety of a colostrum- and Aloe vera-based oral care protocol to prevent and treat severe oral mucositis in patients undergoing hematopoietic stem cell transplantation: a single-arm phase II study
- PMID: 35922679
- PMCID: PMC9463213
- DOI: 10.1007/s00277-022-04934-4
Efficacy and safety of a colostrum- and Aloe vera-based oral care protocol to prevent and treat severe oral mucositis in patients undergoing hematopoietic stem cell transplantation: a single-arm phase II study
Abstract
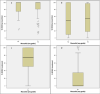
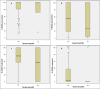
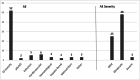
Oral mucositis is one of the worst effects of the conditioning regimens given to patients undergoing hematopoietic stem cell transplantation. It is characterized by dry mouth, erythema, mucosal soreness, ulcers, and pain, and it may impact patient outcomes. Bovine colostrum and Aloe vera contain a wide variety of biologically active compounds that promote mucosal healing. A non-randomized phase II study was designed to assess the safety and efficacy of a combined bovine colostrum and Aloe vera oral care protocol to prevent and to treat severe oral mucositis in transplant patients. Two commercially available products were given to patients in addition to the standard protocol: Remargin Colostrum OS® mouthwash and Remargin Colostrum Gastro-Gel® taken orally. Forty-six (78.0%) patients experienced oral mucositis, 40 (67.8%) developed mild-moderate forms, and 6 (10.2%) severe ones. Comparing the study group's outcomes with those of a homogeneous historical control group, severe oral mucositis decreased significantly (10.2% vs. 28.4%; P < 0.01), as did its duration (0.5 ± 1.9 vs. 1.5 ± 3.0 days; P < 0.01). Febrile neutropenia episodes (69.5% vs. 95.1%; P < 0.01) and duration (4.0 ± 4.7 vs. 6.2 ± 4.5 days; P < 0.01) also decreased. These findings show that the experimental protocol seems effective in preventing severe forms of oral mucositis. However, a randomized controlled trial is necessary to confirm this.
Keywords: Aloe vera; Colostrum; Conditioning regimen; Hematopoietic stem cell transplantation; Oral mucositis; Oral toxicity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Maziarz R (2015) Overview of hematopoietic stem cell transplantation. In: Maziarz R, Slater S (eds) Blood and marrow transplant handbook Springer Cham 10.1007/978-3-319-13832-9_1
-
- Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A, Gratwohl M, Frauendorfer K, Niederwieser D, Horowitz M, Kodera Y. Worldwide Network of Blood and Marrow Transplantation Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;28(30316):1617–24. doi: 10.1001/jama.2010.491. - DOI - PMC - PubMed
-
- Zulu S, Kenyon M (2018) Principles of conditioning therapy and cell infusion. In: Kenyon M, Babic A (eds) The European blood and marrow transplantation textbook for nurses: under the auspices of EBMT [Internet] Cham (CH): Springer Chapter 6 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources