The in-vivo dynamics of Plasmodium falciparum HRP2: implications for the use of rapid diagnostic tests in malaria elimination
- PMID: 35922803
- PMCID: PMC9351188
- DOI: 10.1186/s12936-022-04245-z
The in-vivo dynamics of Plasmodium falciparum HRP2: implications for the use of rapid diagnostic tests in malaria elimination
Abstract
Background: Rapid diagnostic tests (RDTs) that rely on the detection of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) have become key tools for diagnosing P. falciparum infection. The utility of RDTs can be limited by PfHRP2 persistence, however it can be a potential benefit in low transmission settings where detection of persistent PfHRP2 using newer ultra-sensitive PfHRP2 based RDTs can serve as a surveillance tool to identify recent exposure. Better understanding of the dynamics of PfHRP2 over the course of a malaria infection can inform optimal use of RDTs.
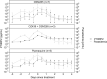
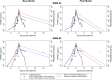
Methods: A previously published mathematical model was refined to mimic the production and decay of PfHRP2 during a malaria infection. Data from 15 individuals from volunteer infection studies were used to update the original model and estimate key model parameters. The refined model was applied to a cohort of patients from Namibia who received treatment for clinical malaria infection for whom longitudinal PfHRP2 concentrations were measured.
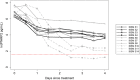
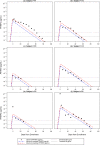
Results: The refinement of the PfHRP2 dynamic model indicated that in malaria naïve hosts, P. falciparum parasites of the 3D7 strain produce 33.6 × 10-15 g (95% CI 25.0-42.1 × 10-15 g) of PfHRP2 in vivo per parasite replication cycle, with an elimination half-life of 1.67 days (95% CI 1.11-3.40 days). The refined model included these updated parameters and incorporated individualized body fluid volume calculations, which improved predictive accuracy when compared to the original model. The performance of the model in predicting clearance of PfHRP2 post treatment in clinical samples from six adults with P. falciparum infection in Namibia improved when using a longer elimination half-life of 4.5 days, with 14% to 67% of observations for each individual within the predicted range.
Conclusions: The updated mathematical model can predict the growth and clearance of PfHRP2 during the production and decay of a mono-infection with P. falciparum, increasing the understanding of PfHRP2 antigen dynamics. This model can guide the optimal use of PfHRP2-based RDTs for reliable diagnosis of P. falciparum infection and re-infection in endemic settings, but also for malaria surveillance and elimination programmes in low transmission areas.
Keywords: Antigen dynamics; Elimination and surveillance; Histidine rich protein; Plasmodium falciparum; Rapid diagnostic tests.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
Similar articles
-
Implications of Parasites Lacking Plasmodium falciparum Histidine-Rich Protein 2 on Malaria Morbidity and Control When Rapid Diagnostic Tests Are Used for Diagnosis.J Infect Dis. 2017 Apr 1;215(7):1156-1166. doi: 10.1093/infdis/jix094. J Infect Dis. 2017. PMID: 28329034
-
Investigation of Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions and performance of a rapid diagnostic test for identifying asymptomatic malaria infection in northern Ethiopia, 2015.Malar J. 2022 Mar 4;21(1):70. doi: 10.1186/s12936-022-04097-7. Malar J. 2022. PMID: 35246151 Free PMC article.
-
Modelling the dynamics of Plasmodium falciparum histidine-rich protein 2 in human malaria to better understand malaria rapid diagnostic test performance.Malar J. 2012 Mar 19;11:74. doi: 10.1186/1475-2875-11-74. Malar J. 2012. PMID: 22423618 Free PMC article.
-
Force Protection Risks in AFRICOM, INDOPACOM and SOUTHCOM Due to Rapid Diagnostic Test Failures for Falciparum Malaria, 2016-2022.MSMR. 2023 Oct 1;30(10):7-11. MSMR. 2023. PMID: 37963222 Review.
-
Genetic diversity and deletion of Plasmodium falciparum histidine-rich protein 2 and 3: a threat to diagnosis of P. falciparum malaria.Clin Microbiol Infect. 2019 May;25(5):580-585. doi: 10.1016/j.cmi.2018.09.009. Epub 2018 Sep 27. Clin Microbiol Infect. 2019. PMID: 30267926 Review.
Cited by
-
Characterisation of Plasmodium vivax lactate dehydrogenase dynamics in P. vivax infections.Commun Biol. 2024 Mar 22;7(1):355. doi: 10.1038/s42003-024-05956-6. Commun Biol. 2024. PMID: 38519588 Free PMC article.
-
Persistence of Plasmodium falciparum HRP-2 antigenaemia after artemisinin combination therapy is not associated with gametocytes.Malar J. 2022 Dec 6;21(1):372. doi: 10.1186/s12936-022-04387-0. Malar J. 2022. PMID: 36474274 Free PMC article. Clinical Trial.
-
Exploring Biomarkers for Malaria: Advances in Early Detection and Asymptomatic Diagnosis.Biosensors (Basel). 2025 Feb 12;15(2):106. doi: 10.3390/bios15020106. Biosensors (Basel). 2025. PMID: 39997008 Free PMC article. Review.
-
Aptamers as innovative tools for malaria diagnosis and treatment: advances and future perspectives.Biol Methods Protoc. 2025 Mar 27;10(1):bpaf025. doi: 10.1093/biomethods/bpaf025. eCollection 2025. Biol Methods Protoc. 2025. PMID: 40223817 Free PMC article. Review.
-
Post-treatment duration of positivity for standard and ultra-sensitive Plasmodium falciparum antigen-based rapid diagnostic tests, a cohort study from a low-endemic setting in Namibia.EBioMedicine. 2025 Jan;111:105489. doi: 10.1016/j.ebiom.2024.105489. Epub 2024 Dec 9. EBioMedicine. 2025. PMID: 39657364 Free PMC article.
References
-
- Cook J, Xu W, Msellem M, Vonk M, Bergstrom B, Gosling R, et al. Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis. 2015;211:1476–1483. doi: 10.1093/infdis/jiu655. - DOI - PMC - PubMed
-
- Hsiang MS, Ntshalintshali N, Kang Dufour MS, Dlamini N, Nhlabathi N, Vilakati S, et al. Active case finding for malaria: a 3-year national evaluation of optimal approaches to detect infections and hotspots through reactive case detection in the low-transmission setting of Eswatini. Clin Infect Dis. 2020;70:1316–1325. doi: 10.1093/cid/ciz403. - DOI - PMC - PubMed

