Neurogenesis of the scallop Azumapecten farreri: from the first larval sensory neurons to the definitive nervous system of juveniles
- PMID: 35922810
- PMCID: PMC9347173
- DOI: 10.1186/s12983-022-00468-7
Neurogenesis of the scallop Azumapecten farreri: from the first larval sensory neurons to the definitive nervous system of juveniles
Abstract
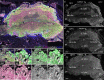
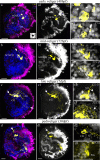
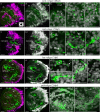
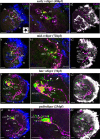
Background: Scallops are among the best-studied bivalve mollusks. However, adult nervous system and neurogenesis studies of scallops are limited. Here, we studied the localization of neurotransmitters (serotonin/5-HT, FMRFamide, catecholamines) in adult ganglia and larvae of Azumapecten farreri using histochemical and immunohistochemical methods.
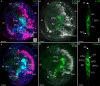
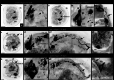
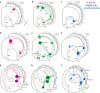
Results: We found peptide FMRFamide in all adult scallop ganglia, whereas 5-HT-like immunoreactive (lir) somata were exclusively detected in the cerebropleural, pedal, and accessory ganglia. Scallop larval neurogenesis starts with the emergence of the 5-HT-lir neurons, which are part of the apical organ (AO) at the early veliger stage. Near the AO, paired anlagen of cerebral ganglion (CG) developed. 5-HT-lir neurites of the CG innervate the velum, ventral, and dorsal parts of the larva at the late veliger stage. Scallop pediveligers possess 5-HT-lir CG, pleural ganglia, and immunopositive signals in the developing enteric nervous system. FMRFamide-lir is first detected in dorsal, ventral, and AO cells of early veligers. Later, FMRFamide-lir extends to the visceral nervous cord, all ganglia, as well as in the enteric nervous system in pediveligers. Catecholaminergic neurons are detected near the larval mouth, in the vellum, and in the stomach in veligers.
Conclusions: We described the distribution of neurotransmitters of the ganglia in adult scallops and the larval neurodevelopment in A. farreri. Immunostaining of neurotransmitters showed that the gross anatomy of adult scallop ganglia, in general, is similar to that in other bivalves, but complicated by the complexity of the structure of the ganglia and the appearance of additional ganglia not described in other molluscs. A comparison of larval neuromorphology suggests that 5-HT-lir structures are more conservative than FMRF-lir structures in Bivalvia. Notably, the latter are much more distributed in scallop A. farreri larvae than in other studied bivalves.
Keywords: Bivalve; Catecholamines; FMRFamide; Ganglia; Larvae; Neurogenesis; Neuromorphology; Serotonin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Comparative Neuroanatomy of Pediveliger Larvae of Various Bivalves from the Sea of Japan.Biology (Basel). 2023 Oct 17;12(10):1341. doi: 10.3390/biology12101341. Biology (Basel). 2023. PMID: 37887051 Free PMC article.
-
Nervous system development in the Pacific oyster, Crassostrea gigas (Mollusca: Bivalvia).Front Zool. 2018 Apr 11;15:10. doi: 10.1186/s12983-018-0259-8. eCollection 2018. Front Zool. 2018. PMID: 29681988 Free PMC article.
-
Towards a ground pattern reconstruction of bivalve nervous systems: neurogenesis in the zebra mussel Dreissena polymorpha.Org Divers Evol. 2018;18(1):101-114. doi: 10.1007/s13127-017-0356-0. Epub 2018 Jan 18. Org Divers Evol. 2018. PMID: 31258414 Free PMC article.
-
Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri.Int J Mol Sci. 2022 Feb 11;23(4):2027. doi: 10.3390/ijms23042027. Int J Mol Sci. 2022. PMID: 35216143 Free PMC article.
-
Peripheral sensory neurons govern development of the nervous system in bivalve larvae.Evodevo. 2019 Sep 12;10:22. doi: 10.1186/s13227-019-0133-6. eCollection 2019. Evodevo. 2019. PMID: 31528326 Free PMC article. Review.
Cited by
-
First characterization of the nuclear receptor superfamily in the Mediterranean mussel Mytilus galloprovincialis: developmental expression dynamics and potential susceptibility to environmental chemicals.Philos Trans R Soc Lond B Biol Sci. 2024 Mar 25;379(1898):20220500. doi: 10.1098/rstb.2022.0500. Epub 2024 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38310933 Free PMC article.
-
The unique biology of catch muscles: insights into structure, function, and robotics innovations.Front Bioeng Biotechnol. 2025 Apr 16;13:1478626. doi: 10.3389/fbioe.2025.1478626. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40309505 Free PMC article. Review.
-
Comparison of neurogenesis in bivalves with different types of development.Sci Rep. 2024 Aug 22;14(1):19495. doi: 10.1038/s41598-024-67622-5. Sci Rep. 2024. PMID: 39174570 Free PMC article.
-
Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia.Int J Mol Sci. 2023 Jan 7;24(2):1202. doi: 10.3390/ijms24021202. Int J Mol Sci. 2023. PMID: 36674710 Free PMC article. Review.
-
Comparative Neuroanatomy of Pediveliger Larvae of Various Bivalves from the Sea of Japan.Biology (Basel). 2023 Oct 17;12(10):1341. doi: 10.3390/biology12101341. Biology (Basel). 2023. PMID: 37887051 Free PMC article.
References
-
- Wilkens LA, Ache BW. Visual responses in the central nervous system of the scallop. Pecten ziczac. Experientia. 1977; 33:1338–1340 https://books.google.de/books?id=vhGdBAAAQBAJ&dq=wilkens+ache+1977&sourc...
LinkOut - more resources
Full Text Sources

