Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses
- PMID: 35922830
- PMCID: PMC9351251
- DOI: 10.1186/s40168-022-01317-9
Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses
Abstract
Background: The cervicovaginal (CV) microbiome is highly associated with vaginal health and disease in both pregnant and nonpregnant individuals. An overabundance of Gardnerella vaginalis (G. vaginalis) in the CV space is commonly associated with adverse reproductive outcomes including bacterial vaginosis (BV), sexually transmitted diseases, and preterm birth, while the presence of Lactobacillus spp. is often associated with reproductive health. While host-microbial interactions are hypothesized to contribute to CV health and disease, the mechanisms by which these interactions regulate CV epithelial function remain largely unknown.
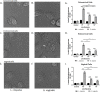
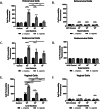
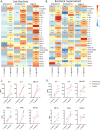
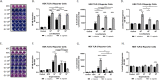
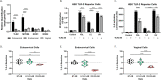
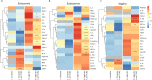
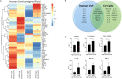
Results: Using an in vitro co-culture model, we assessed the effects of Lactobacillus crispatus (L. crispatus) and G. vaginalis on the CV epithelial barrier, the immune mediators that could be contributing to decreased barrier integrity and the immune signaling pathways regulating the immune response. G. vaginalis, but not L. crispatus, significantly increased epithelial cell death and decreased epithelial barrier integrity in an epithelial cell-specific manner. A G. vaginalis-mediated epithelial immune response including NF-κB activation and proinflammatory cytokine release was initiated partially through TLR2-dependent signaling pathways. Additionally, investigation of the cytokine immune profile in human CV fluid showed distinctive clustering of cytokines by Gardnerella spp. abundance and birth outcome.
Conclusions: The results of this study show microbe-specific effects on CV epithelial function. Altered epithelial barrier function through cell death and immune-mediated mechanisms by G. vaginalis, but not L. crispatus, indicates that host epithelial cells respond to bacteria-associated signals, resulting in altered epithelial function and ultimately CV disease. Additionally, distinct immune signatures associated with Gardnerella spp. or birth outcome provide further evidence that host-microbial interactions may contribute significantly to the biological mechanisms regulating reproductive outcomes. Video Abstract.
Keywords: Cervix; Epithelial barrier; Gardnerella vaginalis; Inflammation; Lactobacillus crispatus; Preterm birth; TLR2.
© 2022. The Author(s).
Conflict of interest statement
MAE receives salary support from NIH (NIAID, NINR, and NICHD). She is a consultant for MIRVIE. ESF has consulted for Astarte Medical Partners and Enzymetrics Bioscience, Inc. The other authors declare that they have no competing interests.
Figures
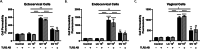
Similar articles
-
Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health.Front Microbiol. 2018 Oct 8;9:2181. doi: 10.3389/fmicb.2018.02181. eCollection 2018. Front Microbiol. 2018. PMID: 30349508 Free PMC article.
-
Gardnerella vaginalis induces matrix metalloproteinases in the cervicovaginal epithelium through TLR-2 activation.J Reprod Immunol. 2022 Aug;152:103648. doi: 10.1016/j.jri.2022.103648. Epub 2022 May 23. J Reprod Immunol. 2022. PMID: 35679790 Free PMC article.
-
Lactobacillus crispatus CCFM1339 Inhibits Vaginal Epithelial Barrier Injury Induced by Gardnerella vaginalis in Mice.Biomolecules. 2024 Feb 18;14(2):240. doi: 10.3390/biom14020240. Biomolecules. 2024. PMID: 38397477 Free PMC article.
-
The Female Vaginal Microbiome in Health and Bacterial Vaginosis.Front Cell Infect Microbiol. 2021 Apr 7;11:631972. doi: 10.3389/fcimb.2021.631972. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33898328 Free PMC article. Review.
-
Research Progress on the Correlation Between Gardnerella Typing and Bacterial Vaginosis.Front Cell Infect Microbiol. 2022 Mar 25;12:858155. doi: 10.3389/fcimb.2022.858155. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402309 Free PMC article. Review.
Cited by
-
Species-level resolution for the vaginal microbiota with short amplicons.mSystems. 2024 Feb 20;9(2):e0103923. doi: 10.1128/msystems.01039-23. Epub 2024 Jan 26. mSystems. 2024. PMID: 38275296 Free PMC article.
-
Host-microbiome interactions in distinct subsets of preterm labor and birth.iScience. 2023 Oct 28;26(12):108341. doi: 10.1016/j.isci.2023.108341. eCollection 2023 Dec 15. iScience. 2023. PMID: 38047079 Free PMC article.
-
Unlocking the Interactions Between the Whole-Body Microbiome and HPV Infection: A Literature Review.Pathogens. 2025 Mar 18;14(3):293. doi: 10.3390/pathogens14030293. Pathogens. 2025. PMID: 40137778 Free PMC article. Review.
-
Integrative Analysis of Shared Pathogenic Genes and Potential Mechanisms in Gardnerella vaginalis and Persistent HPV16 Infection.Mediators Inflamm. 2025 Jun 5;2025:2582989. doi: 10.1155/mi/2582989. eCollection 2025. Mediators Inflamm. 2025. PMID: 40510586 Free PMC article.
-
Extracellular vesicles from vaginal Gardnerella vaginalis and Mobiluncus mulieris contain distinct proteomic cargo and induce inflammatory pathways.NPJ Biofilms Microbiomes. 2024 Mar 21;10(1):28. doi: 10.1038/s41522-024-00502-y. NPJ Biofilms Microbiomes. 2024. PMID: 38514622 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

