Topical administration of the secretome derived from human amniotic epithelial cells ameliorates psoriasis-like skin lesions in mice
- PMID: 35922852
- PMCID: PMC9351215
- DOI: 10.1186/s13287-022-03091-9
Topical administration of the secretome derived from human amniotic epithelial cells ameliorates psoriasis-like skin lesions in mice
Abstract
Background: Psoriasis is a chronic inflammatory skin disease. Tissue stem cells have exhibited a therapeutic effect on psoriatic mice. However, the therapeutic effect of topical administration of the secretome derived from tissue stem cells on psoriasis has not been reported.
Methods: The secretome from human amniotic epithelial cells (AEC-SC) and human umbilical cord mesenchymal stem cells (UMSC-SC) was topically administrated on the back of imiquimod-induced psoriasis-like mice. Subsequently, we observed the skin lesions and skin inflammation of psoriasis-like mice. Next, we further analyzed the paracrine factors in AEC-SC and UMSC-SC by protein chips. Lastly, the effect of the crucial paracrine factor was investigated by imiquimod-induced psoriasis-like mice.
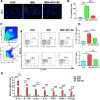
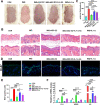
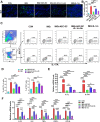
Results: We found that AEC-SC had a better therapeutic effect on attenuating psoriasis-like skin lesions including skin scales, skin redness and skin thickness than UMSC-SC, and it had a better regulatory effect on keratinocyte hyperproliferation and altered differentiation. Thus, we focused on AEC-SC. Further study showed that AEC-SC reduced the infiltration of neutrophils and interleukin-17-producing T cells. Next, the analysis of AEC-SC with protein chip revealed that the levels of anti-inflammatory factor interleukin-1 receptor antagonist (IL-1ra) were much higher in AEC-SC compared to that in UMSC-SC. More importantly, the beneficial effect of AEC-SC on psoriasis-like skin lesions and skin inflammation of mice were significantly impaired when neutralizing with IL-1ra antibody, while the recombinant human IL-1ra showed a less protective effect than AEC-SC.
Conclusions: The present study demonstrated that AEC-SC could efficiently ameliorate psoriasis-like skin lesions and skin inflammation and IL-1ra plays an essential role. Therefore, topical administration of AEC-SC may provide a novel strategy for treating psoriasis-like inflammatory skin diseases.
Keywords: AEC-SC; IL-1ra; Psoriasis; Skin inflammation; Skin lesions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Butyrate receptor HCAR2/GPR109A controls imiquimod-induced psoriasis-like skin inflammation.J Immunol. 2025 Aug 1;214(8):2029-2040. doi: 10.1093/jimmun/vkaf069. J Immunol. 2025. PMID: 40434072
-
Topical astilbin ameliorates imiquimod-induced psoriasis-like skin lesions in SKH-1 mice via suppression dendritic cell-Th17 inflammation axis.J Cell Mol Med. 2022 Feb;26(4):1281-1292. doi: 10.1111/jcmm.17184. Epub 2022 Jan 12. J Cell Mol Med. 2022. PMID: 35023281 Free PMC article.
-
Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis.Int J Nanomedicine. 2018 Jul 20;13:4189-4206. doi: 10.2147/IJN.S165966. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30057446 Free PMC article.
-
Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T cell-derived IL-17.Pharmacol Res. 2018 Mar;129:443-452. doi: 10.1016/j.phrs.2017.11.012. Epub 2017 Nov 14. Pharmacol Res. 2018. PMID: 29155016
-
RNA-Based Antipsoriatic Gene Therapy: An Updated Review Focusing on Evidence from Animal Models.Drug Des Devel Ther. 2024 Apr 23;18:1277-1296. doi: 10.2147/DDDT.S447780. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38681207 Free PMC article. Review.
Cited by
-
Multimodal Function of Mesenchymal Stem Cells in Psoriasis Treatment.Biomolecules. 2025 May 19;15(5):737. doi: 10.3390/biom15050737. Biomolecules. 2025. PMID: 40427630 Free PMC article. Review.
-
Amniotic Membrane and Amniotic Epithelial Cell Culture.Methods Mol Biol. 2024;2749:135-149. doi: 10.1007/978-1-0716-3609-1_13. Methods Mol Biol. 2024. PMID: 38133781
-
Efficient Treatment of Psoriasis Using Conditioned Media from Mesenchymal Stem Cell Spheroids Cultured to Produce Transforming Growth Factor-β1-Enriched Small-Sized Extracellular Vesicles.Int J Stem Cells. 2024 Nov 30;17(4):407-417. doi: 10.15283/ijsc24089. Epub 2024 Oct 14. Int J Stem Cells. 2024. PMID: 39396918 Free PMC article.
-
The epidermal integrin-mediated secretome regulates the skin microenvironment during tumorigenesis and repair.Matrix Biol. 2024 Dec;134:175-183. doi: 10.1016/j.matbio.2024.11.002. Epub 2024 Nov 2. Matrix Biol. 2024. PMID: 39491760 Review.
-
Human Amniotic Epithelial Stem Cells Alleviate Autoimmune Premature Ovarian Insufficiency in Mice by Targeting Granulosa Cells via AKT/ERK Pathways.Stem Cell Rev Rep. 2024 Aug;20(6):1618-1635. doi: 10.1007/s12015-024-10745-z. Epub 2024 Jun 4. Stem Cell Rev Rep. 2024. PMID: 38831179 Free PMC article.
References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, on behalf of the Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Investig Dermatol. 2013;133:377. doi: 10.1038/jid.2012.339. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

