Macrophage-mediated tissue response evoked by subchronic inhalation of lead oxide nanoparticles is associated with the alteration of phospholipases C and cholesterol transporters
- PMID: 35922858
- PMCID: PMC9351260
- DOI: 10.1186/s12989-022-00494-7
Macrophage-mediated tissue response evoked by subchronic inhalation of lead oxide nanoparticles is associated with the alteration of phospholipases C and cholesterol transporters
Abstract
Background: Inhalation of lead oxide nanoparticles (PbO NPs), which are emitted to the environment by high-temperature technological processes, heavily impairs target organs. These nanoparticles pass through the lung barrier and are distributed via the blood into secondary target organs, where they cause numerous pathological alterations. Here, we studied in detail, macrophages as specialized cells involved in the innate and adaptive immune response in selected target organs to unravel their potential involvement in reaction to subchronic PbO NP inhalation. In this context, we also tackled possible alterations in lipid uptake in the lungs and liver, which is usually associated with foam macrophage formation.
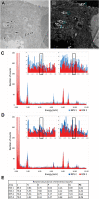
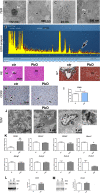
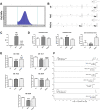
Results: The histopathological analysis of PbO NP exposed lung revealed serious chronic inflammation of lung tissues. The number of total and foam macrophages was significantly increased in lung, and they contained numerous cholesterol crystals. PbO NP inhalation induced changes in expression of phospholipases C (PLC) as enzymes linked to macrophage-mediated inflammation in lungs. In the liver, the subchronic inhalation of PbO NPs caused predominantly hyperemia, microsteatosis or remodeling of the liver parenchyma, and the number of liver macrophages also significantly was increased. The gene and protein expression of a cholesterol transporter CD36, which is associated with lipid metabolism, was altered in the liver. The amount of selected cholesteryl esters (CE 16:0, CE 18:1, CE 20:4, CE 22:6) in liver tissue was decreased after subchronic PbO NP inhalation, while total and free cholesterol in liver tissue was slightly increased. Gene and protein expression of phospholipase PLCβ1 and receptor CD36 in human hepatocytes were affected also in in vitro experiments after acute PbO NP exposure. No microscopic or serious functional kidney alterations were detected after subchronic PbO NP exposure and CD68 positive cells were present in the physiological mode in its interstitial tissues.
Conclusion: Our study revealed the association of increased cholesterol and lipid storage in targeted tissues with the alteration of scavenger receptors and phospholipases C after subchronic inhalation of PbO NPs and yet uncovered processes, which can contribute to steatosis in liver after metal nanoparticles exposure.
Keywords: Cholesterol metabolism; Inhalation; Lead oxide nanoparticles; Liver macrophages; Lung macrophages.
© 2022. The Author(s).
Conflict of interest statement
The authors declared that there is no conflict of interest.
Figures





Similar articles
-
Impact of acute and subchronic inhalation exposure to PbO nanoparticles on mice.Nanotoxicology. 2018 May;12(4):290-304. doi: 10.1080/17435390.2018.1438679. Epub 2018 Feb 15. Nanotoxicology. 2018. PMID: 29447049
-
Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs.Part Fibre Toxicol. 2017 Dec 21;14(1):55. doi: 10.1186/s12989-017-0236-y. Part Fibre Toxicol. 2017. PMID: 29268755 Free PMC article.
-
The effects of nano-sized PbO on biomarkers of membrane disruption and DNA damage in a sub-chronic inhalation study on mice.Nanotoxicology. 2020 Mar;14(2):214-231. doi: 10.1080/17435390.2019.1685696. Epub 2019 Nov 15. Nanotoxicology. 2020. PMID: 31726900
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
Cited by
-
Toxic Kidney Damage in Rats Following Subchronic Intraperitoneal Exposure to Element Oxide Nanoparticles.Toxics. 2023 Sep 19;11(9):791. doi: 10.3390/toxics11090791. Toxics. 2023. PMID: 37755801 Free PMC article.
-
Selenium Nanoparticles in Protecting the Brain from Stroke: Possible Signaling and Metabolic Mechanisms.Nanomaterials (Basel). 2024 Jan 11;14(2):160. doi: 10.3390/nano14020160. Nanomaterials (Basel). 2024. PMID: 38251125 Free PMC article. Review.
-
Study of the Embryonic Toxicity of TiO2 and ZrO2 Nanoparticles.Micromachines (Basel). 2023 Jan 31;14(2):363. doi: 10.3390/mi14020363. Micromachines (Basel). 2023. PMID: 36838065 Free PMC article.
-
Subchronic Inhalation of TiO2 Nanoparticles Leads to Deposition in the Lung and Alterations in Erythrocyte Morphology in Mice.J Appl Toxicol. 2025 Jun;45(6):1004-1018. doi: 10.1002/jat.4759. Epub 2025 Feb 11. J Appl Toxicol. 2025. PMID: 39933250 Free PMC article.
References
-
- Areecheewakul S, Adamcakova-Dodd A, Haque E, Jing X, Meyerholz DK, O’Shaughnessy PT. Time course of pulmonary inflammation and trace element biodistribution during and after sub-acute inhalation exposure to copper oxide nanoparticles in a murine model. Part Fibre Toxicol. 2022;19:40. doi: 10.1186/s12989-022-00480-z. - DOI - PMC - PubMed
-
- Asgharian B, Price OT, Oldham M, Chen LC, Saunders EL, Gordon T, Mikheev VB, Minard KR, Teeguarden JG. Computational modeling of nanoscale and microscale particle deposition, retention and dosimetry in the mouse respiratory tract. Inhal Toxicol. 2014;26(14):829–842. doi: 10.3109/08958378.2014.935535. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

