Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings
- PMID: 35922965
- PMCID: PMC9465188
- DOI: 10.1111/jcmm.17497
Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings
Abstract
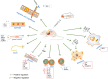
Mesenchymal stem cell (MSC) therapy is considered a new treatment for a wide range of diseases and injuries, but challenges remain, such as poor survival, homing and engraftment rates, thus limiting the therapeutic efficacy of the transplanted MSCs. Many strategies have been developed to enhance the therapeutic efficacy of MSCs, such as preconditioning, co-transplantation with graft materials and gene modification. Hepatocyte growth factor (HGF) is secreted by MSCs, which plays an important role in MSC therapy. It has been reported that the modification of the HGF gene is beneficial to the therapeutic efficacy of MSCs, including diseases of the heart, lung, liver, urinary system, bone and skin, lower limb ischaemia and immune-related diseases. This review focused on studies involving HGF/MSCs both in vitro and in vivo. The characteristics of HGF/MSCs were summarized, and the mechanisms of their improved therapeutic efficacy were analysed. Furthermore, some insights are provided for HGF/MSCs' clinical application based on our understanding of the HGF gene and MSC therapy.
Keywords: HGF gene-modified MSCs (HGF/MSCs); clinical application; hepatocyte growth factor (HGF); mesenchymal stem cells (MSCs); therapeutic efficacy.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

