Ultrasound biomicroscopy for the assessment of early-stage nonalcoholic fatty liver disease induced in rats by a high-fat diet
- PMID: 35923118
- PMCID: PMC9532208
- DOI: 10.14366/usg.21182
Ultrasound biomicroscopy for the assessment of early-stage nonalcoholic fatty liver disease induced in rats by a high-fat diet
Abstract
Purpose: The aim of this study was to assess the ability of ultrasound biomicroscopy (UBM) to diagnose the initial stages of nonalcoholic fatty liver disease (NAFLD) in a rat model.
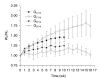
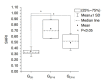
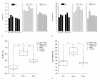
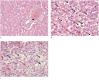
Methods: Eighteen male Wistar rats were allocated to control or experimental groups. A high-fat diet (HFD) with 20% fructose and 2% cholesterol, resembling a common Western diet, was fed to animals in the experimental groups for up to 16 weeks; those in the control group received a regular diet. A 21 MHz UBM system was used to acquire B-mode images at specific times: baseline (T0), 10 weeks (T10), and 16 weeks (T16). The sonographic hepatorenal index (SHRI), based on the average ultrasound image gray-level intensities from the liver parenchyma and right renal cortex, was determined at T0, T10, and T16. The liver specimen histology was classified using the modified Nonalcoholic Steatohepatitis Clinical Research Network NAFLD activity scoring system.
Results: The livers in the animals in the experimental groups progressed from sinusoidal congestion and moderate macro- and micro-vesicular steatosis to moderate steatosis and frequent hepatocyte ballooning. The SHRI obtained in the experimental group animals at T10 and T16 was significantly different from the SHRI of pooled control group. No significant difference existed between the SHRI in animals receiving HFD between T10 and T16.
Conclusion: SHRI measurement using UBM may be a promising noninvasive tool to characterize early-stage NAFLD in rat models.
Keywords: Animal models; Dietary cholesterol; High-fat diet; Nonalcoholic fatty liver disease; Ultrasonography.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- World Health Organization . World health statistics 2020: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2020.
-
- Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

